Everything posted by studiot
-
Is foundational physics stuck?
Newton and Einstein had the advantage of practical work already done by someone else. The point of this practical work was that it did not conform to the then thinking. The influence of both their insights not only answered the original problem that arose from observation not matching theoretical expectation, but spread far and wide into many other disciplines. That is why they were so important. The practical came first the insight followed on. So there is little point complaining that theorists have not come up with anything new. Again from the UK point of view. A century and more ago the UK government began creating research establishments at University and above level. These embraced diverse subjects from agriculture and forestry to hydraulics research to military to building and many more. In the last 50 or so years, successive governments have been busy closing them down and/or selling them off but generally pulling out of this sort of thing. We are steadily loosing the capability to do the experiments that throw up the big questions of "why does this happen this way, and what else can I do with it. ?" Love it. +1
-
Equal fractions
It's the sort of thing that is being studied in what's called (Higher) Arithmetic these days. The Higher Arithmetic Davenport Cambridge university press
-
Equal fractions
It's not a trick question, its designed to test the limits of computers. Wolfram Alpha gave up when I asked it. There are 7 factors, all different. One of them is very large itself. Of course they are all prime. Isn't that a requirement for factors ? The second one is 353
-
Is foundational physics stuck?
I can't agree with Sabine or her harangue. Nor was I impressed by the camerawork continually switching viewpoints. I don't agree with her premise that there has been no progress in the last 50 years. Just that she is looking in the wrong place for it. Yes I am sure there are problems in academia, but I have emboldened your second question as it contains where I think some of those problems lie. "Specifically" is the key to me. Specialisation. Not just in Science, but since you ask, specifically in Physics. Here I have to confess to being partly a victim of my own comment since I can really only speak authoritatively for the UK and there is a much wider world out there. That is not to say I think everything in the garden is rosy, but there are also external factors in play, the largest being political interference.
-
Equal fractions
Yes 17 is the only 2 digit factor (in decimal) The next one is 3 digits and the one after that 6 digits.
-
Equal fractions
yes starting with the shortest one is good.
-
Two studies find SARS-CoV-2 virus becoming resistant to antiviral drugs used to treat patients
thanks for that expert opinion. +1
-
Equal fractions
Some official current practice sites https://mathshub.thirdspacelearning.com/ https://assets.publishing.service.gov.uk/media/5a7da548ed915d2ac884cb07/PRIMARY_national_curriculum_-_Mathematics_220714.pdf factorise 2484 + 1
-
Equal fractions
When I first started arithmetic (we put sums on the fron of our exercise books) we didn't write mathematical expressions like or do brackets or equivalence classes 567 + 123 = We laid out our 'sums' like this (sorry I can't get this site to represent a continuous line under the sum) [math]\begin{array}{*{20}{c}} 5 & 6 & 7 & {} \\ 1 & 2 & 3 & {} \\ - & - & - & + \\ 6 & 9 & 0 & {} \\ \end{array}[/math] and [math]\begin{array}{*{20}{c}} 5 & 6 & 7 & {} \\ 1 & 2 & 3 & {} \\ - & - & - & - \\ 4 & 4 & 4 & {} \\ \end{array}[/math] Muliplication and division was laid out similarly. After the idea of fractions was introduced as already noted in a previous post we found that fractions as the ratio of two numbers became messy and this led on to the idea of decimal fractions, laid out in the same way but now with a whole number part a decimal part and a decimal point. I don't know if they still do this in school but I do worry about loss of the development from arithmetic into algebra in these days where no one actually needs to do calculations this way.
-
1. Sub Quantum Echo Particles...(SQEP's) & Sub Quantum Echo Particle Kinetic Resonance Flux
https://simple.wikipedia.org/wiki/Island_of_stability https://www.thoughtco.com/island-stability-discovering-new-superheavy-elements-4018746
-
1. Sub Quantum Echo Particles...(SQEP's) & Sub Quantum Echo Particle Kinetic Resonance Flux
There are a little over 80 stable or long life radioactive naturally occurring elements. There are a little over 10 extra elements which are shorter life radioactive and can occcur naturally on Earth or elsewhere. The rest of the known elements up to the 118 currently known have very short lives indeed so we don't find them naturally, we only find them in reactions as intermediates towards some other element. So in the last 50 years we have discovered something like 50 previously unknown elements. Some of these were predicted before actual discovery. In the same way calculation suggests there is an 'island of comparative stability' between elements 125 and 135, if we can find them. 150 is a nice round number that may be realised within the next 50 years.
-
1. Sub Quantum Echo Particles...(SQEP's) & Sub Quantum Echo Particle Kinetic Resonance Flux
The second line is a bit Irish isn't it ? The pattern I am thinking is All material matter is made of ↓ Either pure substance or a mixture of pure substances. ↓ Pure substances are made of ↓ Molecules which are all the same ↓ Molecules are made of one or more atoms of the same or different kinds ↓ There are many (millions) of different kinds of molecule but ↓ There are only about 150 kinds of atom. ↓ Each different atom is unique to a particular element ↓ Each element is represented once only by its own symbol in the periodic table.
-
A photon as a 'twist' in space
As a guide here are a couple of pages of very good advice of how to develop your theory of how your medium can support and transmist EM waves. From Optical Physics Lipson and Lipson Cambridge university press.
-
1. Sub Quantum Echo Particles...(SQEP's) & Sub Quantum Echo Particle Kinetic Resonance Flux
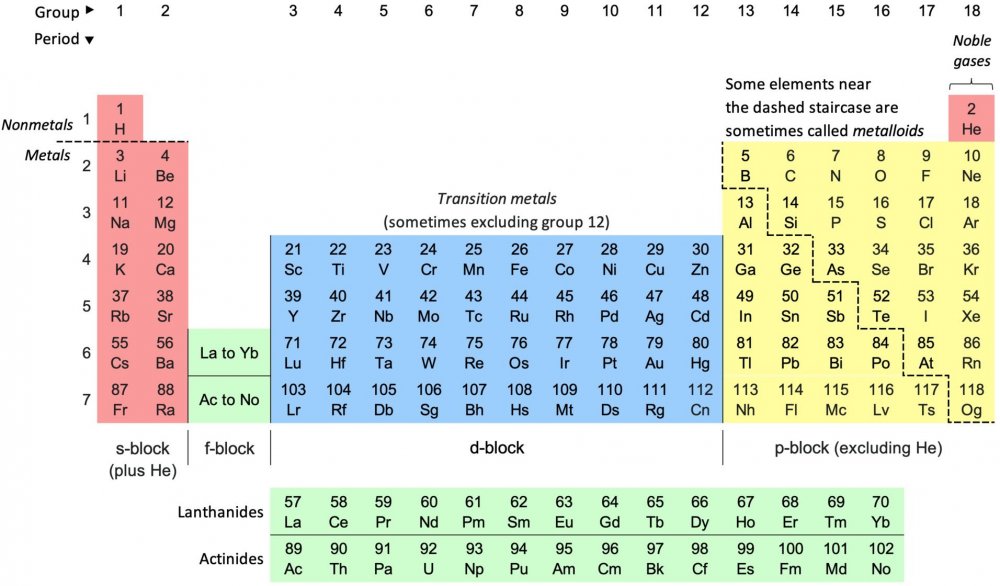
Here is wikipedia's period table and a link to the Royal Society of Chemistry interactive one which is a good one to play with https://www.rsc.org/periodic-table Wikipedia uses the term noble gases (an old fashioned term) for what I call the inert gases. Helium (He) is the one with the smallest molecule and appears at the top of the extreme right hand side of the table. The combined two pink columns on the left and 6 yellow block on the right comprise my 0ctet (8) original table. Don't worry about the blue and green for the momment. I just picked oxygen as an example I thought you might be familiar with. The point of valence is that you can't just make up combinations od different atoms (ie different elements) there are rule of combination some rules tell you if they combination is feasible. Valence tells you how much of each will combine if the combination is feasible. Keep asking questions that's the way to understanding.
-
Two studies find SARS-CoV-2 virus becoming resistant to antiviral drugs used to treat patients
Is this fake news for disruption purposes ? Remdesivir is still the treatment of choice in the UK https://www.nhs.uk/medicines/remdesivir-veklury/how-and-when-to-have-remdesivir/ Perhaps @CharonY can advise us reliably ?
-
1. Sub Quantum Echo Particles...(SQEP's) & Sub Quantum Echo Particle Kinetic Resonance Flux
A molecule has to be made of at least one element. Helium is an element that does not bond and is called an inert gas. A helium molecule contains exactly one atom of helium and nothing else. By contrast an oxygen molecule contains 2 atoms of the element oxygen only and an ozone molecule contains 3 atoms of oxygen only. A carbon dioxide molecule contains one atom of carbon and two atoms of oxygen. A water molecule contains one atom of oxygen and two atoms of hydrogen. Air is a mixture of nitrogen molecules, oxygen molecules and carbon dioxide molecules and a very small percentage of other molecules Can you see the pattern beginning to develop?
-
1. Sub Quantum Echo Particles...(SQEP's) & Sub Quantum Echo Particle Kinetic Resonance Flux
This is Chemistry, not Physics. Before doing valency, you need to do substances, elements, atoms, molecules and chemical bonds. So first we consider pure substances (impure substances are called mixtures) A pure substance has every part with its constituents in the same proportions or percentages. This is known as the Law of Constant Proportions. So every particle of common salt is 38.8% sodium and 61.2% chlorine. The smallest particle of a substance is called a molecule. Molecules are made of only a small number of basic substances called elements. The smallest particle of an element is called an atom. The molecules of some elements appear as single atoms others appear as two or more atoms joined together. When atoms join together the resulting molecules are held together by chemical bonds. There are three basic types of chemical bond Ionic Covalent Metallic All types of bond are the result of activities by the electrons in the atoms. For our purposes we may consider that these electrons are held in 'shells', one shell inside the other and each shell can accomodate a specific number of electrons. The number of electrons in an electrically neutral atom is fixed and equal to the atomic number of that element. As the atomic number increases the elements fill up the shells, starting with the innermost and proceeding to the next shell only when the innermost is full up. The first or innermost shell holds only 2 electrons. The next innermost shell can hold 6 electrons. So electrons for any element with 3 or more electrons will have 1,2,3,4,5 or 6 electrons in the second or outer shell Shell 1 plus shell 2 can hold 8 electrons This accounts for there being 8 basic columns in the periodic table with the most common elements in the first 'period' or horizontal row. An atom can change its number of electrons by becoming an ion . It can gain or loose one or more electrons an so become a positive ion ( Cation) or negative (anion) Such ions can interact with other ions electrostatically to form an ionic bond. The valency of such a bond will be equal to the number of electrons gained or lost so with single negative charge bonding ionically to a single positive charge, as in the salt example the valency is one. We do not however normally get single molecules of salt, but larger crystals being a three dimensional array of positive sodium ions and negative chlorine ions. So the crystal is still ionically bonded but each individual ion now connects to a surrounding group of the other ions and distributes a little bit of that valency to each. So in ionic crystals we have a different situation and the surrounding number of ions around each ion is called its coordination number. For pretty pictures see link. https://en.wikipedia.org/wiki/Coordination_number Now I said that 8 is a special number. It is true that atoms without 8 electrons in their shell can share electrons with electrons in another atom. When they share they are held together by a basic bond called a covalent bond. Here the covalent valency is the number of electrons it can share that is all the electrons in the incomplete outer shell. So Carbon, atomic number 6, has 4 electrons in its outer shell and has a valency of 4 as it can gain a share in up to 4 electrons. Finally and only for completeness some elements (metals) can join together in what ammounts to giant crystal molecules with each metallic atom contributing an electron to a common pool which then belongs to the wole lump of metal holding it together.
-
Equal fractions
OK personal interest. That's great. Thanks. Even equivalence classes have to start with a bunch (I won't say set) of rules. And these rules define what can and cannot be done with the resulting numbers and expressions. Furthermore these rules have to be learned and accepted. It is also worth noting that some number systems are not susceptible to this analysis. The number systems employed in Polynesian and Australian Aboriginal tribes for instance. Also the Systeme Internationale organisation has recently added 'number' as a fundamental physical property base to add to the original 5 ( mass, length, time, electric current density and illumination).
-
What affordable sheet metal will be resistant to high temperatures without warping?
Yeah you really need a foundry for cast metals, which is why cooker manufacturers, like AGA, often use inserts in the hob. I can see that this is difficult with your diesel flame and fumes. But it is not only the expansion that is important. The heat transfer coefficient is also important. Basically ferrous alloys - iron, steel etc have good coefficients. Fancy steels, especially stainless steels, have significantly poorer coefficients. This is why all good quality pan bases are not solid stainless steel but some sort of sandwhich (often copper based). Otherwise there is not only the heat loss from having to drive the system harder but also the greater danger of burning the contents. Many very thin bottomed pans as susceptible to this burning, if they are made of stainless steel.
-
Early Morning Club
Good Morning. Have you heard of Penrose Tiling https://en.wikipedia.org/wiki/Penrose_tiling Escher https://mathandart.com/blog/escher_and_tessellations/ Tesselations https://www.mathnasium.com/blog/what-is-tessellation-in-math ? You should be able to find many more references for yourself. 😀
-
Equal fractions
One thing to remember is that we do not teach any serious subject all in one go. Mathematics especially requires what I call a 'spiral approach'. Here a very simplified version is first presented, not the whole nine yards (nice expression in English for you). This will tie in with what has gone before and what may be presented in the future as the subject is revisited again and again as we work around the spiral. So in the present context fractions will be naturally introduced after addition, subtraction ,multiplication and division. Note these are treated separately and simply and usually called 'sums' (and in the olden days tables). So a fraction naturally becomes replacing the dots in the division sign with actual numbers. This fits in with your Penrose description and emphasises that it is division being talked about. Which naturally leads to division of pies, apples, sweets whatever. In turn this leads to finding out the difference between dividing a bag of sweets and a single pie. This leads back to proper and improper fractions (and perhaps vulgar fractions). The four arithmetic operations can then be revisited in the light of fractions, leading to the introduction of decimal fractions. Then we have the return to the four operations to work decimal fractions. Looking ahead to secondary school and algebra we are set up to try algebraic fractions and find out why (4-2) / (14-2) is not the same as (2-2) / (7-2. The next bump comes when the teacher needs to keep emphasising that dy/dx is not a fraction, but a complete entity in itself. Having said all this it would be very helpful if you would indicate why you are asking these questions and where you are going with the answers ?
-
Equal fractions
Do you prove Pythagoras every time you use it ? Of course not. The teacher may well explain that if we multiply by 1, which is the same as 1/1 or 2/2 or 3/3 etc we don't change anything. But no suggestion of adding or subtracting would be made . In fact later the same or similar discussion would occur when introducing powers and roots of fractions.
-
Equal fractions
As I recall it was not called cancellation of common factors it was called 'Do the same thing to top and bottom' So either multiply the 2 and 7 by or divide the 4 and 14 by 2, depending upon which way round the question was asked. Often it would be simplify or express in simplest terms 4/14
-
That g__am vignette.
Google vignette is driving me mad and away. How do fix it so every second attempt to move page, post, change forum section... does not lead to blankout.
-
Equal fractions
Clearly your second childhood has arrived. Only children will say Mom my piece of pie is smaller than his. 😀