Everything posted by John Cuthber
-
Messages to the president...
Mr President; someone has suggested a name for the new Ballroom. https://en.wikipedia.org/wiki/Orangery
-
Messages to the president...
Please spare a thought for the Translator at Davos who had to translate "If it was not for me, you would all be speaking German" into German. Also, well done to Democratic president Harry S Truman for seeing this potential issue in 1951 and striking a deal with Greenland.
-
Today I Learned
I must have been busy 9 years ago so I missed the opportunity to post this. https://www.youtube.com/watch?v=YaGdwfykYGY
-
Trump Says U.S. Will ‘Run’ Venezuela
Could someone do me a favour please? Tell Trump that North Sea oil has pretty much run out, so there's no point in invading the UK. Thanks. (on behalf of about 70 million of us) And , now for the science part... When North sea oil came on stream, Venezuelan oil actually became more valuable. As you say, Venezuelan crude is heavy. On the other hand, North Sea crude is much lighter. By blending them, the oil companies could use the mixture in their refineries that were designed for use on Arabian oil which is intermediate between the two.
-
Trump Says U.S. Will ‘Run’ Venezuela
It's clear that Venezuela has been gravely mismanaged for decades. And now, they will get "run" by a man who managed to bankrupt six casinos and thinks you can drop drug prices by 800%. I guess the good news is that the people are used to being shafted. It's also fair to say that, if Trump's claims about drugs are honest, the USA will no longer have a drug problem. Is anyone taking bets on that ?
-
Optimal location ?
The sag left to right will be bigger than the sag from top to bottom anyway, because it's a longer unsupported span. If it matters, you should address that first.
-
Silicone remover / solvent suggestions ?
The only thing I know of that reliably removes silicones is a saturated solution of potassium permanganates and sodium hydroxide in water. It attacks glass slowly and almost everything else quickly. You are likely to end up using a pot scourer.
-
"Wave if you're human"
Yes, but "So improper wording rules by accepting the wrong slang." doesn't seem to parse in English. Did you not see the irony?
- "Wave if you're human"
-
Messages to the president...
Apparently the "Trump said he "talked to the president of Puerto Rico"" story is false but the Trump's remarks to the 2017 Values Voters Summit, at which he misspoke, saying he had met with the "President of the Virgin Islands". story is true...
-
USA vs Europe
Comparing things to an institution which has not existed for about a third of a century is... odd. On December 25, 1991, the Soviet hammer and sickle flag lowered for the last time over the Kremlin, thereafter replaced by the Russian tricolor. I own a 3 necked flask. It's just a bit of glass, but it is banned in Texas. You can sell any cucumber you like in Europe, provided that it's fit to eat, or clearly marked as not being so. You seem to have fundamentally failed to grasp the difference between US and UK (and, to an extent, the rest of Europe's) law. Americans often point out that the UK has no "bill of rights". That is true. We don't need one. We effectively have a "bill or wrongs". We have a long list of laws which tell you what you are forbidden to do (and owning a gun is not on that list, no matter what Faux news told you) Anything which is not forbidden is permitted by default. Part of the reason that many UK houses are relatively small is that they are old. Another is that they are not made of cardboard.
-
What are the best two words and the worst two words in the English language
hyphenated non-hyphenated
-
What if Putin used a tactical nuke in Ukraine?
"why would you want to irradiate the land you want to occupy?" It may be dawning on Putin that he's not going to occupy much.
-
Michelson–Morley experiment limit.
This is a 3 page thread that should have been 2 posts. Why don't we all stop here, and pretend that it was? The question (in spite of a typo) was fully answered in the first reply.
-
Home-made poisons for ants...
Regardless of the scale, surface tension is weaker than a solid gut wall. Borates are not very toxic acutely in humans. Not sure about insects.
-
Home-made poisons for ants...
I never studied ant anatomy, or really studied that of humans. But I know that we can burp, and I don't see why ants would not be able to do so. Exploding ants seems unlikely. However, if they eat a significant amount of sodium bicarbonate, that will throw their electrolyte balance out of kilter. Also, in many countries, compounding your own unlicensed insecticides is illegal.
-
Estimated Time of Death
Fraud is a matter for the police, and they know the answers to the questions you asked.
-
Trump is discussing deporting US citizens: “Get them the hell out”
I'm not sure there's a useful definition of "Okay" which includes the situation in the USA. I note with some amusement that Trump is "only" a US citizen by birthright. It would be perfectly possible to legally remove his citizenship under the laws he's planning to introduce. Seems fair. Maybe deport him to an El Salvadoran jail?
-
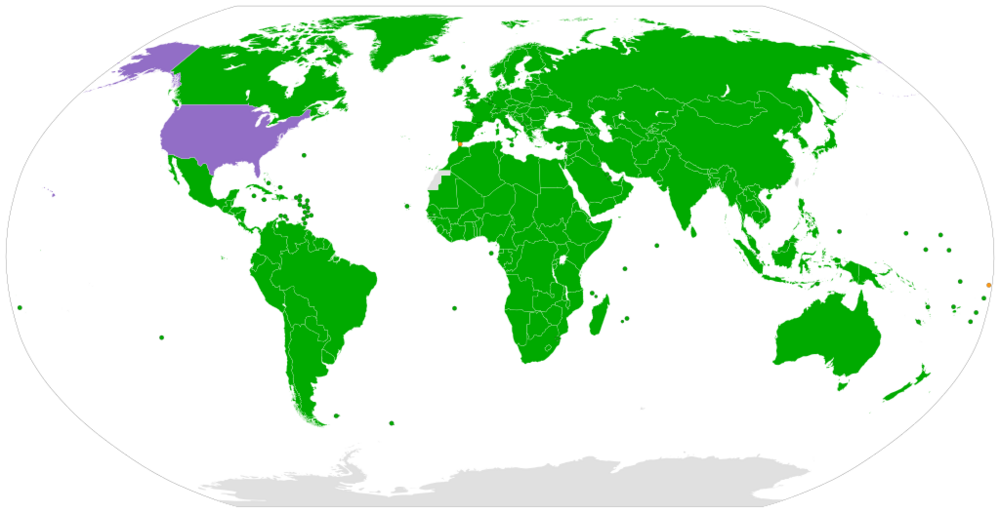
Trump is discussing deporting US citizens: “Get them the hell out”
This map indicates in green, the states where it is forbidden to deprive a person of statehood if that person is a child. And it shows an uncivilised country in purple. https://en.wikipedia.org/wiki/Convention_on_the_Rights_of_the_Child#/media/File:Convention_on_the_Rights_of_the_Child.svg
-
The "Third Condiment Mystery"
It may suggest that to you, but not to me. I know that maltodextrin is used as a sorbent I'd expect any acetate of maltodextrin to behave a bit like either https://en.wikipedia.org/wiki/Sucrose_octaacetate which is very bitter Or https://en.wikipedia.org/wiki/Cellulose_acetate Which is tasteless. That's interesting. I wonder if it was chemistry or microbiology. If you add an inoculum of acetic acid bacteria to wine, you will get vinegar. It does seem redundant to have a solid form of vinegar in the same cruet as the liquid- but it's not impossible. It's possible that the filling for the "third pot in the set" was left to the choice of the household (or their butler).
-
The "Third Condiment Mystery"
I don't know if it was in common use. I have seen it listed as a flavouring. But the point is that it wasn't hard to prove that it was not A spot of googling would have told you that, and I think you owe the OP an apology.
-
The "Third Condiment Mystery"
https://en.wikipedia.org/wiki/Sodium_diacetate
-
What Emily Lime prefers
EVE and OTTO.
-
What Emily Lime prefers
I gather she has a friend named Eve and another called Otto. For some reason she seems to prefer to see then written in all caps.
-
Optimal location ?
Does this help? https://en.wikipedia.org/wiki/Airy_points