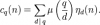Chemistry
Subforums
-
Chemistry with inorganic compounds.
- 1k posts
-
All chemistry involving organic compounds (those with C-H bonds).
- 882 posts
2901 topics in this forum
-
Okay so I want to know everything about Covalent Bonds and Ionic bonds. And what is the differences between them, and how you identify which molecules have either covalent or ionic bonds, etc. Thanks for telling me.
-
0
Reputation Points
- 9 replies
- 3k views
-
-
I read that an electron in a molecular anti-bonding orbital is at a higher energy compared to the atomic orbital than an electron in a bonding orbital however it does not explain why an electron in an anti-bonding orbital is at a higher energy. See attached image. Without going into advanced chemical principles could someone explain why this is the case? Thanks.
-
0
Reputation Points
- 1 reply
- 1.1k views
-
-
Hi! Has anyone already tried the new Air Liquide (link removed by moderator) App? It could be usefull to convert units...
-
0
Reputation Points
- 1 reply
- 915 views
-
-
nitrous oxide..NO2 automotive is NO2S sulfer is added. i need to find a way to get nitrous oxide that is at 32*f to maintain 950 psi in a tank with a siphon tube. at 32* NO2 is at roughly 460psi. when used in racing, the nitrous is heated to arounf 92* to acheive 950ish psi. i need a non flamable solution and somthing that wont hurt the NO2 properties. my theory is colder nitriuos=smaller molicules smaller means more in a certain size space. more is good thank you for reading i hope i made it clear enough.. also the 950 psi could be in a 2nd pusher type bottle if needed
-
0
Reputation Points
- 0 replies
- 786 views
-
-
why do oil makes paper translucent even water can't do so ?
-
0
Reputation Points
- 3 replies
- 1.5k views
-
-
Hey guys, I'm doing basic chemistry schooling and i'm a little confused about on of my assignment questions... I would love some insight! So the question is: What ions would/would not be easily detected with Silver Nitrate... In the prac, I mixed the AgNo3 with the anions, so would I assume that mixing the AgNo3 with anions is preferable because the silver forms a visable precipitate where it wouldnt with a Cation? Im not sure how the positive and negatives factor here... I understand the AgNo3 is an ionic compound + and - and the anions are - But we used ionic compounds for the Cations too (NaOH and NH4OH) I'm just lost as to why AgNo3 in…
-
0
Reputation Points
- 1 reply
- 2.8k views
-
-
How do I know the state of molecular compounds? I know that they can be solid liquid or gas, but how do I know which? For example H and O are gases at SATP but as a molecular compund they are a liquid. Also for acids, my textbook says that acids are solids liquids or gases as pure substances but then it says they are aqueous. What am I missing? Thanks
-
0
Reputation Points
- 7 replies
- 28k views
- 1 follower
-
-
-
I have engaged myself a bit with people that do fireplay for scening on two continents and is trying to understand littlebit more about two liquied used. When I was in northamerica I have seen people use 70% Isopophyl alcohol, refered as something very cheap to buy in any store. Believe they called it "rubbing alcohol" In Scandinavia where I come from 95% denaturated ethonol is what seems to be used for this purpose , as it is what is avaliable cheap and easy. I have some questions 1. Do these liquids have different flame temperature 2. Do any of these liquieds burn as "Cool flame" 3. Is there any other interesting practical difference between the…
-
0
Reputation Points
- 3 replies
- 1.4k views
-
-
House, please I need ideas on how to convert graphite to diamond. I am working on a project on this. Your ideas are highly welcome.
-
0
Reputation Points
- 2 replies
- 1.2k views
-
-
-
Hello,I am needing a little help in the process of making aqua regia,Is this correct?1 pound of nitrate,1 quart of distilled water to dissolve nitrate,1 quart of muratic acid,3 quarts of sufferic acid to make 1 aqua regia bath.If this is correct how many ounces of computer fingers( gold content ) will it dissolve?Thank you in advance very much for helping me.
-
0
Reputation Points
- 1 reply
- 1.6k views
- 1 follower
-
-
As a science student I almost feel foolish for even asking this, but do acids like those seen in movies such as Cube or Aliens actually exist outside of Hollywood? For those of you who haven't seen Cube, in one of the scenes, a 'prisoner' is sprayed in the face with some sort of extremely corrosive liquid, presumably an acid, that literally results in his head being practically hollowed out it is so corrosive. Within the span of several seconds or maybe a minute, the acid had completely eaten into his brain and reduced his entire head to little more than a hollow skull. Now I'm 99.9% sure that an acid as powerful as this is a good example of Hollywood ludicrousy, but …
-
0
Reputation Points
- 1 reply
- 1.6k views
-
-
IN THE MATTER OF PRODUCT KEYS, FADING OR WEARING OFF, SCIENCE COULD BE THE ANSWER TO A PROBLEM THAT COSTS MANY DIYS NEW SOFTWARE EXPENSE. COULD THERE BE A METHOD OF VIEWING THE CODE WITH FILTERS OR CHEMICAL ENHANCEMENT. I FEEL VIOLATED BY THOSE WHO WOULD PUT CODES IN AN AREA THAT'S KNOWN TO PROBABLE WEAR. ANY IDEAS?
-
0
Reputation Points
- 1 reply
- 1.2k views
- 2 followers
-
-
I'm stumped! I've never seen this until today. How is ithe following possible. http://www.sootoday.com/content/news/full_story.asp?StoryNumber=50683%20
-
0
Reputation Points
- 2 replies
- 1.3k views
-
-
why tubes get blackned at the ends after some times and which substance does it ?
-
0
Reputation Points
- 13 replies
- 1.9k views
- 1 follower
-
-
I was making stew just the other day, and being a novice at it, ended up with globs of fat in my stew. Quick search through the internet found that skimming the surface with a leaf of lettuce would help, as fat would stick to the leaf. Tried it, and it worked... not too well, but enough to get by. Just curious as to what it is about lettuce leaf that lends to it such property. -CGorge
-
0
Reputation Points
- 0 replies
- 745 views
-
-
I'm trying to implement a H2S (hydrogen sulfide) measurement for thermal water in an in situ sample collection by an environmental microbiology and microbial ecology laboratory. I need to stabilize the H2S in the water sample to measure the H2S levels later. Due to the higly volatility of H2S, I'm evaluating (and understand the theory of) the methods of blue methylene or diamine; but I don't know the practical limitations for an in situ sampling neither what is the proper container for water with volatile H2S.
-
0
Reputation Points
- 1 reply
- 1.5k views
- 1 follower
-
-
So i bought a childrens chemistry set and a basic book on chemistry; The book describes the basis of chemistry such as exchanging electrons and bonding at the atomic level as well as some explanation of how chemical equations work and some examples of chemical extractions and different forms of reacting chemicals. I'd like to know where to find a good introductory website or book that shows you some good reactions to do with a basic set of chemicals, maybe walking you through the chemical equations and showing where to go from the new chemical thats been created. Also i could write a list of chemicals i currently have and you could just suggest example experiments…
-
0
Reputation Points
- 0 replies
- 1k views
-
-
Hey all! Hope you'll bear with me. I'm an adult who finally has an opportunity to re-invest and further his science educaiton. My last formal educaiton was University level General Chemistry about 15 years ago. I'm hoping to be able to go back and do some organic chemistry courses in the near future. So in the mean time I'm doing some self-study. So I was experimenting with some copper sufate in an aquaeous solution. I then added some aluminum and precipitate out the copper, and then I outdid myself and decanted the aluminum sulfate and added magnesium, precipitating out what I assume is aluminum powder. My focus is on understanding the exact nature of the reactions go…
-
0
Reputation Points
- 1 reply
- 1.4k views
-
-
basicly I have been lookinging into smoke bombs and this mixture seems the most easy and effective(smoke effective). But In High stress situation fiddling with a match box to light the wick is too obvious to your opponent, and I have yet to see a pin mechanism that does not use a whole machbox per bomb (which is not very conservitive for supplize). so can you explain how one would manage to make a dependable impact smoke bomb using KNO3 and sugar. thanks and while i'm on this would brown sugar produce more thick smoke? Its all about makeing a smoke screen in this one.
-
0
Reputation Points
- 2 replies
- 1.6k views
-
-
I am aware that when plants get exposed to gasoline fumes in air such as plants nearby roads, plants in nearby school traffic area, they seem to die out more quickly and i affected in a bad way one way or another. Now i am interested in finding out how to prevent the plants from dying when exposed to the aromatic hydrocarbons produced by cars, buses etc.... Is there any specific way of doing this, (maybe if not preventing, just improving the sustainability of the plants life under these conditions would also be considered). i would greatly appreciate the help Thank you
-
0
Reputation Points
- 6 replies
- 2k views
-
-
I was wondering of someone can clear up the difference between heat and enthalpy?
-
0
Reputation Points
- 3 replies
- 1.3k views
- 1 follower
-
-
I am doing a project called Adopt-An-Element. Part of the project is to create an advertisement for my element which includes the elements name, symbol, atomic number and mass, cost and an advertising slogan that describes one or more of it's important uses. I am having trouble coming up with an advertising slogan for my element, Chromium. Any ideas?
-
0
Reputation Points
- 4 replies
- 2.8k views
-
-
Hello and thanks for visiting this topic. i am looking for a formula to make Lacquer Thinner for thinning Lacquer Paints for Cars and Trucks. i have been told that Main and most important property in it will be ACETONE? i know and i am sure there are people in this forum with lots and lots of knowledge on this regards. My problem is i cannot just add acetone and few other chemicals to make up this product, i need to be 100% sure what needs to go in by what quantity and therefore i will highly appreciate if someone can help me with this formula. My kind regards
-
0
Reputation Points
- 1 reply
- 2.6k views
-