Everything posted by studiot
-
Double displacement of Sodium/potassium carbonate and calcium hydroxcide
Electrolysis preparation is apparantly more common in the US than the UK. I do not know about Europe or other places.
-
Special Relativity Paradox
I'm sorry, what did you want to say ? An explanatory diagram would help greatly her. Observed ? Observed by whom, in what frame ? greater charge density ? greater than what ? length contraction again observed by whom in what frame ?
-
Uncountability of the Cantor's set
The [0,1] map is probably the standard way of doing this but there are others eg in ternary.
-
Quantum fields and consciousness (split from Nothing and The Creation)
Just a human thought about consciousness. We are now beginning to probe and perhaps understand the interactive relationships between plants, particularly trees, and soil mycelium. Since this is new territory there is no reason to suppose that such relationships can be set in the same terms as ones we understand much better and know far more about. So I must agree with Gees. I suggest we need new terminology, concepts and all the apparatus for these new lines of enquiry. Until these are established we can expect heated discussions set in inappropriate terms.
-
Double displacement of Sodium/potassium carbonate and calcium hydroxcide
Solubility constant for calcium carbonate is 1000 time less than for calcium hydroxide, so yes you will remove some calcium carbonate and be left with a sodium carbonate solution, sightly enriched with sodium hydroxide. How are you going to unmix that ?
-
First post, hello, I have a lot of questions.
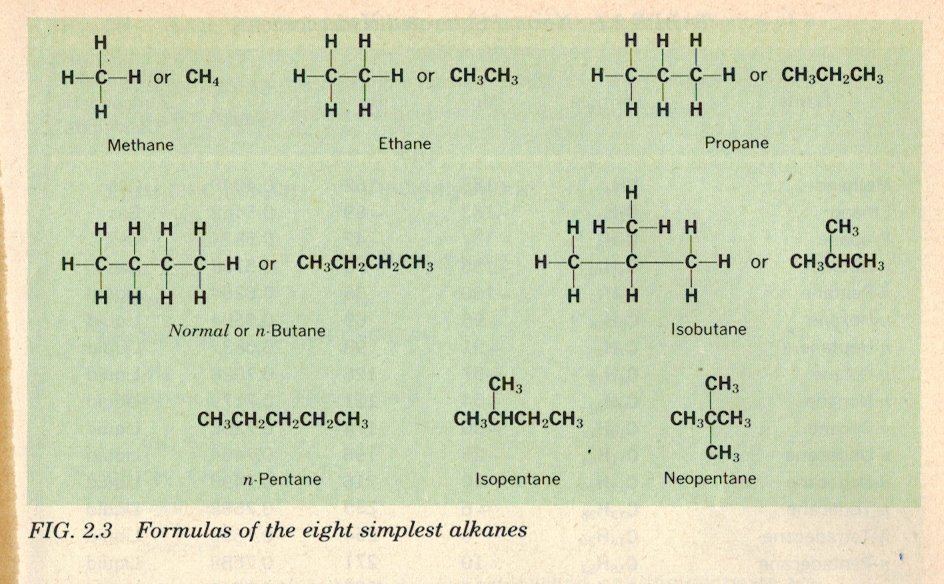
Who are 'they' ? We try to be more specific in Science. But this story is a good example of how Science works. Science does not work on proof. It works by using the best explanations available for known observations. If new observations become available that show different results then a scientist has choices. He can propose a new explanation that explains both the old and the new. He can propose that the new observations are the result of different circumstance or different mechanisms and thus require a new hypothesis. He can propose new observatins to test his new theory or hypothesis. Either way Science moves on, improving on the original and waiting for the next new set of different observations. In fact this third period yielded thousands of new compounds all obeying the valency rules I outlined. Ethane is a good example of this as it is a member of what is known as a homologous series of gases , called the alkanes. But what they did not know before 1900 was why the valency rules are as they are. The answer to this will come in the fourth period after the structure of the atom was discovered and is a subject for tomorrow. Suffice to say for now that these hooks are now called chemical bonds. Finally my apologies to anyone reading this, in my last post i got the date of quantivalence wrong. It should be 1865, not 1869. The first English use of valence was in 1869.
-
First post, hello, I have a lot of questions.
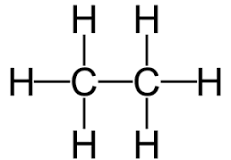
Hopefully your continued exposure to chemical selected names and terms is beginning to bear fruit. So let us carry on with unravelling the history of the subject. The ancient Greeks thought there were four elements, Earth, Water Air and Fire and that all substances were made up from these. In fact they used a different word and the word element came into English from the Latin elementum. Furthermore the concept was very vague in detail and one Greek in particular (Democritus) asked the important question. "What happens if you cut a substance in half, then in half again and in half again and so on ?" He proposed that you would eventually reach a stage where the substance became indivisible and called this piece atomos from where we get the English word atom. This situation continued until the late 17 hundreds when Dalton revived the twin concepts and included the new question "If you can cut substances apart, how can you put them together ?" In his words he described atoms as "All atoms of the same element are alike, globular and all of the same magnitude, but atoms of different elements have different weights." Thus moving atoms from substances to elements and making the distinction. It should be noted that 'weights' were not measured in pounds and ounces or kilogrammes. Hydrogen was give the weight exactly 1 unit and other elements were measured as multiples (including decimal fractions) of this. These weights were called atomic weights. This was a great step forward but it did not explain how or why atoms could be combined in 'fixed proportions' to form substances they could split up. Atoms could not be split up i.e. were indivisible. These insights plus the growing list of elements enabled the first versions of the periodic table to be drawn up. But they were wrong because they placed elements in order of increasing atomic weight, which led to inconsistencies in the chemical properties compared to their placement in 'the table'. The table is called periodic because these properties occur at regular spacing when the elements are placed in the proper order. They had not yet addressed the second question "How can you put them togerther?" Then in 1869 Hofmann, then working in england, coined the English word 'quantivalence'. He introduced the concept of Valency or the combining power of atoms and your next equation from chemical mathematics. Atomic weight = Equivalent weight x Valency. This ushered in the third era in the History of Chemistry and led to a new idea - that of the molecule. At that stage, they still though atoms were 'indivisible' they did not know about electrons, protons and neutrons - that comes in the fourth period up to the present day and was largely invetigated by Physicists. So they quickly determined that oxygen has 2 'hooks' , carbon has 4 'hooks' and nitrogen has '3 hooks' and hydrogen has 1 hook. These hooks were also quickly translated into the ubiquitous chemical stick diagrams we still use today. Here is the diagram for 'ethane' where you can quickly see that each carbon is linked by 4 sticks or hooks and each hydrogen is linked by 1 stickk or hook. This is the 'molecule' of pure ethane.
-
Let's play biochemical detective
I am unclear exactly what the question is here. However try comparing with other similar compounds, that are better known because they are not so harmful. Key thoughts synthetic (poly) saccharide Non-absorbable interacts with colonic bacteria
-
First post, hello, I have a lot of questions.
Well that is an excellent article. +1 And therein goes the material I was going to use for my next post, so I will just pick out the important points that lead to the conclusion I was going to offer. How we got to our modern view of chemistry can be divided into four broad periods. The ancients began to notice that there were many different substances in the world around them. The substances were different because they had different properties. They were hard or soft, some interacted visibly with other substances. Some did not appear to interact at all and some offered protective qualities for other substances. In particular sometimes winemaking went wrong and an unpleasant sour tasting drink was produced instead of acceptable wine. They did not know that wine had turned to acetic acid but their word for vinegar (which is dilute acetic acid) passed down into the Latin word 'acidus' and from there into English as acid. They would also have noticed other properties of acids such as the sting of formic acid in ant bites and the corrosive effect on the skin. It is not known which hero cook spilled animal fat on the fire and roasted it along with wood ash and then found a soapy blob when the result had cooled. But we think this is how middle eastern civilisations discovered soapmaking this way. The ashes provided an alkaline substance which is breaks down fat, something most acids are unable to do. So their for ashes passed into Arabic, Al-Kali and then into English as alkali. Wood (and other plant ) ashes contain what gardeners and farmers call 'potash' which makes a strong alkali with water that we now call potassium hydroxide. So we have the origins of acids and alkalis. The second historical period when humans were able to refine and classify substances, many of which occurred naturally or as with potash by heating or burning and perhaps then adding water. The addition of water was known as slaking; probably the most known and important product was slaked lime or calcium hydroxide, which formed the basis of Roman cement. To obtain this rocks containing calcium carbonate were heated to obtain what was known a quicklime (which we know as calcium oxide). The was the slaked to produce the hydroxide. Calcium carbonate introduces another acid we call carbonic acid, which is important in environmental chemistry. So during this period many names were introduced that were carried forward to the third period which is for next time. Interestingly these time periods have have been 'telescoping'. The first was measured in thousands of years, the second in hundreds of years, and so on.
-
Any Linux users? Is my bad experience of ubuntu just bad luck?
Good morning Ken. Sorry to hear of your woes. I too had less than good experiences with linux in the early days so I dropped it as I did not want to learn yet another op system / language. To your issue. What version of Windows are you trying to install with ? I wonder if the hidden boot partition introduced in W10 is causing this ?
-
Capitalization of the pronoun 'I'
I thought they were brothers ! 🤑
-
Capitalization of the pronoun 'I'
That i8s the difference between the active voice and the passive voice, not the difference between nominative (subject) and accusative (direct object. For example In December, Fred buried me. Active case Subject - Fred object - me (accusative case of the 1st person singular) In December I was buried by Fred. Passive case Subject - I "Fred buried I" is wrong unless you are in a West Country pub where they do say pharases like "Fred buried I" . But then they also say "Dorset be beautiful" 😀
-
Capitalization of the pronoun 'I'
I would also add that English I follows Latin in that it is always in the nominative case. The accusative case, which is used by some languages is me in English. So It is I is correct It is me is incorrect. Finally the Oxford English does not even offer a word i in the lower case, only the upper case. So I am inclined to agree with genady.
-
Capitalization of the pronoun 'I'
Yes I agree with exchemist that is a very good question. +1 The use of I in capitals goes back a long way, the earliest reference I (😀) can find is Preface to Eneydos 1490 William Caxton Where the English is recognizable. Notes Caxton was a writer as well as a printer. The spellung is original, not my usual typing dislexia. Secondly English followed Latin in many ways for instance using Latin numerals. Think what would happen if we wrote 7 Vii or worse VIi ? Chaucer was to far back and too different to be any help.
-
First post, hello, I have a lot of questions.
That's really good We used to call this activity 'reading around the subject' though nowadays you could call it viewing investigating or whatever. And you are using the chemical vocabulary you are acquiring. 😀 Let's look at the first chemical equation I wrote for you again, but in more detail. Acid + Base = Salt + Water. I didn't mention before that there are many different acids; there are many different bases. The substance that is produced by the acid + base reaction is called a salt. Sodium chloride is also called common salt, but it is just one of many salts that can be produced. Each salt is specific to both the acid and base that react with each other to form the salt. Some salts are water soluble, some are not. The 'or not' is important because it is often useful to find out and eliminate what a sample is not. Silver nitrate is a salt which is water soluble white salt. Silver chloride is a white salt that is not soluble in water. Now you are asking about identification. So an easy first check is to see what it is not i.e. does it dissolve in water ? Well we have seen the result of that experiment so we can se that if the white powder that purports to be silver nitrate dissolves in water it could be correct. But if it does not dissolve the it definitely could not. This kind of thinking is not only important in chemical analysis, but also in geology since it helps determine what substance could or could not be in a given bunch of minerals. It is also important in chemical synthesis - that is finding ways to make a particular chemical substance. Remember our way of making sodium chloride is the Acid + Base = Salt + Water equation or reaction. Hydrochloric acid + Sodium hydroxide = Sodium chloride + Water. But this will not work well for silver to make silver chloride because silver hydroxide is almost insoluble in water. So to make silver nitrate we need a different reaction and obviously with the right acid, this time nitric acid. Here is a short video of how to make silver nitrate by dissolving pure silver in nitric acid (This is an exmaple of an oxidation- reduction or redox reaction already mentioned). I am not suggesting you actually carry it out for safety reasons. After this I think it is time we started putting some flesh on the idea of stuff or matter. As someone with good knowledge of flight and aviation I hope you understand the difference between weight and mass ? If not let us know and we will incorporate that.
-
First post, hello, I have a lot of questions.
H2O is a chemical shorthand formula. It is not an equation. Equations have and equals sign, so some formulae but not all, are also equations. Equations do not need to use shorthand symbols, they can be much easier without when you first start using them. This is why I haven't yet got to symbols, atoms, molecules etc. You need some basic stuff to work with for those symbols to have any meaning. So don't worry there is method in my madness and we will get there. Also I have carfully selected my examples to follow from each other so here goes with some more. There are two videos and one picture this time. Many substances occur naturally in the world around us. You asked where their names came from. As humans began to explore their world they named materials they found around them, particularly those they found to be useful. This naming started thousands of years ago and is still going on today. The result of this is that some chemicals with modern names can be simply refined or extracted from our natural world. Some folks actually make a business of this. However of the 4000 or so minerals that we know of only about 10 are very very common (over 90% of the earth's surface). The rest are uncommon. Even carbon (which is not mentioned in the next video) is only 0.02% of the Earth's crust. So I hope the video will show why I am not launching straight into more advanced chemistry including the periodic table, formulae and so on. These will come, but you need to have a reason to look at them. As I said I am following on from previous posts. The discovery of Hydrogen and oxygen and other gases is fascinating, but they made many mistakes in those days so it is better to wait for these. Finally sliver nitrate. I mentioned that this is the basis of photography so here is a nice short video about this. I live near the Fox-Talbot museum, which is a fascinating place to visit.
-
First post, hello, I have a lot of questions.
No you are not thick and these are basic questions, which as you say, are fundamental. But they are very good questions. Don't worry we are covering these. I hope you are taking in the new terminology. I am trying not to introduce too many new words at once. So I think we are ready for your first chemical equation ACID plus BASE = (makes) SALT plus WATER Note that chemical equations are somewhat different than mathematical ones, although we will come to do some mathematics with them. Such a chemical equation represents a chemical reaction (or in this case a whole class of chemical reactions) So we have Hydrochloric Acid plus Sodium Hydroxide makes Sodium Chloride plus Water. Acids were once called spirits and hydrochloric acid was called spirits of salt. Sodium hydroxide has a common name as caustic soda. So our friend either made the chlorides this way or bought them ready made. I think MigL has described acids quite well for you. if that is not enough please ask for more. The 'salt' created by hydrochloric acid is called a chloride. Why chlorides ? Well most chlorides are (very) soluble in water. That's how the presenter got the solutions. There are always exceptions the main ones being the chlorides of silver, lead and mercury are hardly soluble. So next bit of theory. Solutions are made by dissolving a solute (sugar, salt etc) in a (suitable) solvent. Thinking back to our earlier posts a solution is a mixture because the parts can be separated by mechanical means boiling off the solvent to leave the solute behind. This process is called distillation. Another similar sort of mixture is called a suspension. Here fine particles of one substances is intimately mixed with another substance. The result is called a suspension. This is not solution, although the suspended particles may be recovered by boiling, they may also be recovered by passing the suspension through a sufficiently fine filter. Finally (for this post) we can also separate the solute (or part of it) by a Precipitation Reaction. Here we take a solution of an obviously soluble salt and add a solution of a different salt which will react with the salt already in solution in such a way as to produce a Precipitate. This happens when the added new solution contains ions that are insoluble as a salt of the original solute. As an example we now know that silver chloride is insoluble, and I am now stating (from experience) that silver nitrate is soluble. So if we add a soluble chloride (such as sodium chloride) to a silver nitrate solution we we observe a (white) precipitate - of silver chloride. In effect the sodium and silver exchange their type of salt. This sort of reaction is important in both analytical chemistry and for the preparation of chemical compounds. The silver chloride can be filtered off or used in a second reaction as in the video. We are now building up chemical vocabulary to use in the future.
-
First post, hello, I have a lot of questions.
The flame test I referred to forms the beginnings of most modern spectroscopy, one of the main modern analytical tools. Chemists have much to thank Physicists for in the development of the modern spectrometer. There are various sorts, Infra red, Mass, Ultra violet, etc. These are capable of answering the "How much ?" question and are often fully computerised and automated. So there is a new term here, spectrum, which basically means a range of values. Note also in the flame tests that the presented is using ions, which we will come to. These are ions in solution (dissolved in a solvent) probably the most common way Chemists use ions.
-
First post, hello, I have a lot of questions.
Ok so let us look further into analytical chemistry. Say we have plenty of sample to play with. We are normally interested in the answers to one or two questions or both. The first question we ask is What is in it or what is it made of ? This is called qualitative analysis and is by far the easier of the two. The second question is How much ? This is called quantitative analysis and I will come to that later. So we can look at it and ask Is it a solid, liquid or gas (at room temperature) ? What colour is it ? - Most substances are a white powder, colours are less usual and generally distinctive. Will it dissolve in water, acid, benzene, or other common solvents ? Geologists carry little bottles of acid which test rocks for the presence of calcium (eg limestone, chalk etc). Lavousier may well have tried a flame test, although he would not have the modern advantages in this video from MIT. Note in the video after the introduction the flame colours are shown with the overall flame colour on the left and the different colours making up the overall on the right.
-
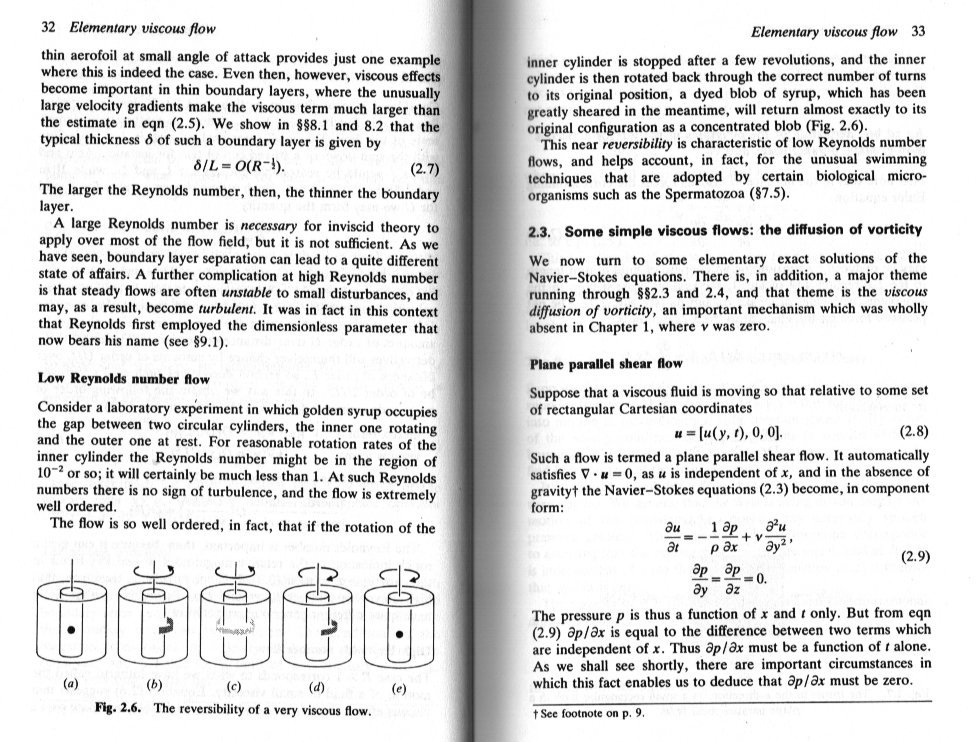
Speculative science questions
Is my screen deficient? Only I can't trace a response to my previous post. Here is some fluid mechanics theory which describes your idea. But you cannot go back before the initial conditions.
-
First post, hello, I have a lot of questions.
Glad you liked it. So I would not start with atoms, molecules, electrons and protons, although you mqy have heard of some of them. This is because we start with our encounter with the surrounding material world. We create words and language to name and describe these things, rather as I have done with iron, wood, concrete. water etc. Then with our scientific hat on we abstract properities, characteristics and abstract descriptions about these same things and how they might be similar or different. We create an abstract model in our heads, computers or on paper. So then we have two worlds a direct material one and an abstract one to deal with and this abstract one is where beginners especially find difficulty. So I am going to suggest you re-read the list of sub caegories of chemistry I mentioned in my first post and we find a few examples in a preferable category to introduce the abstact science of chemistry and its specialist nomenclature. You have already done this to some extent with your question This is the way to keep the theory in touch with the tangible which makes for a more interesting subject experience. So how about you pick one area and I will offer some examples ?
-
This is my documents. Please point out any mistakes and let me know. Please do this to help me, thank you.
Indeed so. +1 Because Your document is not Science. In fact it contains many obvious experimental inaccuracies. Proving anything is not the scientific way. A counterexample may or may not disprove something. Over two and thousand years ago Greek philosophers believed that they could sit in the comfortable villas and dream up hypotheses about how the natural world behaves without ever checking to see if their ideas replicated what actually occurred. They were serious wrong in many many respects and it has taken Science thoudands of years to move on to a better ( as in more successful) approach. Unforunately you appear to be trying to tread the same path as those ancient Greeks. So I seriously you do a couple of things. Firstly find out about the Scientific Method, which drives scientific activity. And also findout the difference between an Hypothesis and a Theory. You can ask here at SF about this, I'm sure Phi is bursting to explain this. Secondly ask about some basic understanding of Science in general and Physics in particular to improve your future thinking.
-
Best Materials for Lightweight Robotic Arm Design?
Good reply +1. Robots are the subject of electromechanical engineering. Is your laboratory some other discipline and you need a robot arm for say moving test tubes ? Or is this an academic electromechanical project ? I ask because if this enquiry is part of a coursework or project work we are only allowed to advise. My thoughts here are that the arm should be hollow, with a sectional material as far as practicable from the holow centre, and preferably longitudinally fluted or ribbed. This will ensure the necessary rigidity as far as possible. The mobile platform should be counterweighted to avoid and tipping when the arm is extended extended past the edge of the platform. A carbon fibre fishing rod will hold 2kg but bend excessively and not be controllable for precise placement. I would suggest that ease of manufacture should be considered, depending upon what you have available. Titanium requires special machines methods. Aluminium is subject to fatigue, depending upon the alloyand is extremely difficult to weld reliably so consider riveted joints. Carbon (or glass) fibre should be laid in mats with the weave laid criss cross to form the hollow tube. The whole process requires curing time etc for the resins so a single arm would take longer to manufacture that metal. This is an important factor in a production line.
-
First post, hello, I have a lot of questions.
OK to start see my reply to this thread Any questions on this ?
-
Fog harvesting could provide water for arid cities
When did you last go up Ben Nevis ? 😀 Dune is Science Fiction which also involved telephathic powers and other stuff. But thanks for the replies guys.