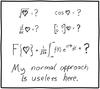Chemistry
Subforums
-
Chemistry with inorganic compounds.
- 1k posts
-
All chemistry involving organic compounds (those with C-H bonds).
- 882 posts
2900 topics in this forum
-
Ok this question is the real BS: The blood alcohol (C2H5OH) level can be determined by titrating a sample of blood plasma with an acidic potassium dichromate solution, resulting in the production of Cr3+(aq) and carbon dioxide. The reaction can be monitored because the dichromate ion (Cr2O72-) is orange in solution, and the Cr3+ ion is green. The unbalanced redox equation is shown below. Cr2O72-(aq) + C2H5OH(aq) → Cr3+(aq) + CO2(g) If 31.05 mL of 0.0600 M potassium dichromate solution is required to titrate 30.0 g of blood plasma, determine the mass percent of alcohol in the blood. I have no clue where to even start. I don't know how to balance that equation, as…
-
0
Reputation Points
- 9 replies
- 5.8k views
-
-
Check out this video: http://ca.youtube.com/watch?v=PTBSp1QvCuw&feature=related Is it real science or pseudo-science? It strikes me as paranoid conspiracy theory. It's about a group of scientists who discovered a cheap and easy way to perform cold fusion but the scientific community refuses to believe their results are legitimate.
-
0
Reputation Points
- 18 replies
- 2.7k views
-
-
would it be possible to react NO2 and CH4 together to form NOC and H20? would you be able to ignite this mixture to give the said products or would this not work?
-
0
Reputation Points
- 6 replies
- 1.4k views
-
-
At my school today, i was doing some Halogen Halide reactions, and also, the reaction of a Halogen with Silver Nitrate. I spilt some of the solution containing Ag+ and Cl- ions on the edge of a test tube (i presume), but didn't notice, and must have come into contact with it. Upon getting home tonight i now have a silver/grey looking patch on my little finger (about the area of a british 5 pence coin), has the Silver Chloride, produced silver due to light exposure, some how in my skin? Is this dangerous? Thanks Alex
-
0
Reputation Points
- 4 replies
- 11.2k views
-
-
Dear Forum Members. Worldwide, the most commonly used fumigant to rid shipping containers of pests is methyl bromide. It is highly efficient at doing this but it is being phased out, worldwide, because it is an ozone layer depleter. Methyl iodide (MeI) is being considered as a substitute fumigation gas. It is equally efficient but has a much shorter atmospheric lifetime (and negligible ozone depletion potential) but is much less volatile. It is proposed to use it in combination with heat treatment where hot air (~45 degrees C) containing about 25g of methyl iodide/cubic m is circulated through the container in a closed system. It is becoming less acceptable to disc…
-
0
Reputation Points
- 8 replies
- 2.6k views
-
-
i've been reading about phenomena such as; relative atomic mass electronegativity density ionisation energies melting point atomic radius electron affinity boiling point Q. which of these are PHYSICAL properties? and which are CHEMICAL properties? Q. what is the difference between a physical property and a chemical property?
-
0
Reputation Points
- 7 replies
- 1.9k views
-
-
Ok i got a question(again)! What are the forces that hold the molecule together to form a liquid? Look up examples of the magnitude of these forces and give value in kj/molecule! Thats the part i am stuck in, how can i possibly know the magnitude or hydrogen bonds, london forces etc. Can anyone help!
-
0
Reputation Points
- 8 replies
- 5.2k views
-
-
Can anyone help me with my research. I'm interested in adsorbing latex particles onto SAM's but I can't find any work on this by anyone else, does anyone know of research on this or something similar?
-
0
Reputation Points
- 4 replies
- 1.1k views
-
-
I saw on wikiHow a pictorial on making "hot ice," and it says, "If you don't have sodium acetate, you can make your own from baking soda and vinegar, but it's time-consuming. Keep adding baking soda to vinegar until it stops fizzing; this reaction yields a diluted solution of sodium acetate and water. Then boil off all of the water to make sodium acetate crystals, which you can treat like the powder as described in the instructions above." What would be the cleanest, most efficient way to make Sodium Acetate? Once it stops bubbling, any extra baking soda might contaminate the desired sodium acetate product. It says to keep adding baking soda, but when the water is…
-
0
Reputation Points
- 8 replies
- 6.4k views
-
-
How does nestle quik dissolve ?http://www.scienceforums.net/forum/images/smilies/rolleyes.gif
-
0
Reputation Points
- 2 replies
- 1.1k views
-
-
Hi everyone, this is my first post on this forum! I've been stalking it for a little while and now I really need some help. I am a biology major and I am currently taking a relatively basic Chem 112 class. However, my professor moves through lecture very quickly and I'm having trouble understanding the first part on chemical kinetics. Also guys, sorry if I don't provide enough information in this post, i can provide it tomorrow, I've had a long day and i'm making this thread as a last resort before i go to bed. my lecture is tomorrow and i'll be talking to my professor then. Anyways, the part i'm stuck on is first, second, third order reactions. I'm pretty much co…
-
0
Reputation Points
- 7 replies
- 2k views
-
-
Ok, so I was thinking. Each element in the universe seems to have a color related to it. I'm sure this is either incorrect, or there are exceptions. Basicly, I would like to know How a specific color portreys itself. I am under the understanding that Color is measures in wavelengths, yes? This means that one color would have the wave lengths closer together then another. This is how we differentiate, correct? So how is it that the reflection off an item, reflects the color of that item? Does the reflection slow the light or something? Ummm, maybe it soaks up some of the energy emitted from the light, leaving a slower wave length? There has to be some …
-
0
Reputation Points
- 6 replies
- 1.8k views
-
-
Hello, I am curious to know if anyone can tell me how to dissolve a block of concrete....? I have about three tons of concrete massed in my back yard, and I hope to simply dissolve it by chemistry. Is there an acid that will break the concrete down over time, say a months time, that would do the trick? Any ideas? Thanks alot!
-
0
Reputation Points
- 2 replies
- 1.7k views
-
-
The issue I want help with. When I mix water with sugar on a 1:1 ratio, it works as an excellent carrier for pigment, and when applied to paper or timber etc., the water evaporates and the sugar acts as a binder and holds the pigment to the surface. IF it is done in a VERY fine layer, it works well, however if the "paint" is applied a little bit on the thick side, the sugar tends to remain tacky - especially in hot or humid environments. It becomes much like an old boiled sweet in ones pocket on a hot sweaty day. This "tackyness" tends to glue or stick anything that is applied to it - and this is the problem. The pages DO stick together. OK so I figure…
-
0
Reputation Points
- 0 replies
- 1.2k views
-
-
I can't figure out what this means. What is it even asking?
-
0
Reputation Points
- 6 replies
- 1.9k views
-
-
What are the properties of H2O molecules that allow them to form the unique geometrical patterns that snowflakes take and how is it done?
-
0
Reputation Points
- 4 replies
- 1.4k views
-
-
This guy was tracing patterns on his t-shirt with ultraviolet LEDs. The shirt was absorbing the light and wherever he traced, it would leave a glowing pattern that would eventually disappear. I asked him what he used and he said he used invisible ink. I ordered some invisible ink of my own and tried it on a shirt but the ink wasn't absorbing the light and leaving glowing trails. The only thing it did was glow if I shined the ultraviolet light over it but it would disappear as soon as I moved the light away or turned it off. Does anyone know what the guy used?
-
0
Reputation Points
- 3 replies
- 1.3k views
-
-
Is all this stuff the same ? Washing soda = laundry soda = sal soda = scotch soda = soda crystals = sodium carbonate = Na2CO3 So many chemicals with multiple 'common' names If yes; here in synonyms, sodium compounds are named, but the formula does not contain sodium ???? http://www.hmdb.ca/metabolites/HMDB03538
-
0
Reputation Points
- 1 reply
- 984 views
-
-
So I'm still VERY new to chemistry, and right now I'm just focusing on different general compounds. However, I still don't quite understand WHY exactly you put different numbers after some elements. Here are just some examples...can someone PLEASE explain to my why these numbers go where they do, and HOW I FIND THEM? Examples: Silver Carbonate - AgCO3 -- I don't understand the 3 Calcium Nitride - Ca3N2 -- I don't understand the 3 and 2 Aluminum Sulfide - Al2S3 -- I don't understand the 2 and 3 Thanks.
-
0
Reputation Points
- 2 replies
- 1.5k views
-
-
My chemistry teacher is helping me make thermite and he is the type that asks a lot of questions and gives out very few answers. so ive still got a lot of questions left before he will okay me to make thermite. i read that thermite used in welding is 25% Al and 75% Fe2O3, is that true and should i use this ratio? i also came across this ratio: 2 mols of Al is equal to 1 mol of Fe. True? know any good ways to make iron oxide (Fe2O3) quick? i found one that sugests taking steel wool and dipping it in vinigear and leaving it in a jar for roughly 15 minutes. good idea?
-
0
Reputation Points
- 13 replies
- 2.3k views
-
-
Can a liquid, gas, or plasma be compressed into a solid if u keep a constant temeperature. I realize the relationship between temp/pressure which is why I am asking this question with the added "constant temperature". Thanks
-
0
Reputation Points
- 5 replies
- 1.4k views
-
-
Does anyone know the name of the chemical which is a colourless dye, until it reacts with the Amino acids on your skin? Which then causes it to turn a purple colour?
-
0
Reputation Points
- 3 replies
- 1.1k views
-
-
change in chemical composition(oxalate ion) of guava during different stages of ripening
-
0
Reputation Points
- 1 reply
- 998 views
-
-
:confused:well i couldnt find anything about Gundanium except the fact that its the universes strongest metal. if anybody can give information about this Gundanium element this please do tell.
-
0
Reputation Points
- 11 replies
- 8.3k views
-
-
Someone asked me recently: What is the basic principle behind the layout of the traditional Periodic Table? I can not answer this question in one short sentence. Can you?
-
0
Reputation Points
- 13 replies
- 2.8k views
-