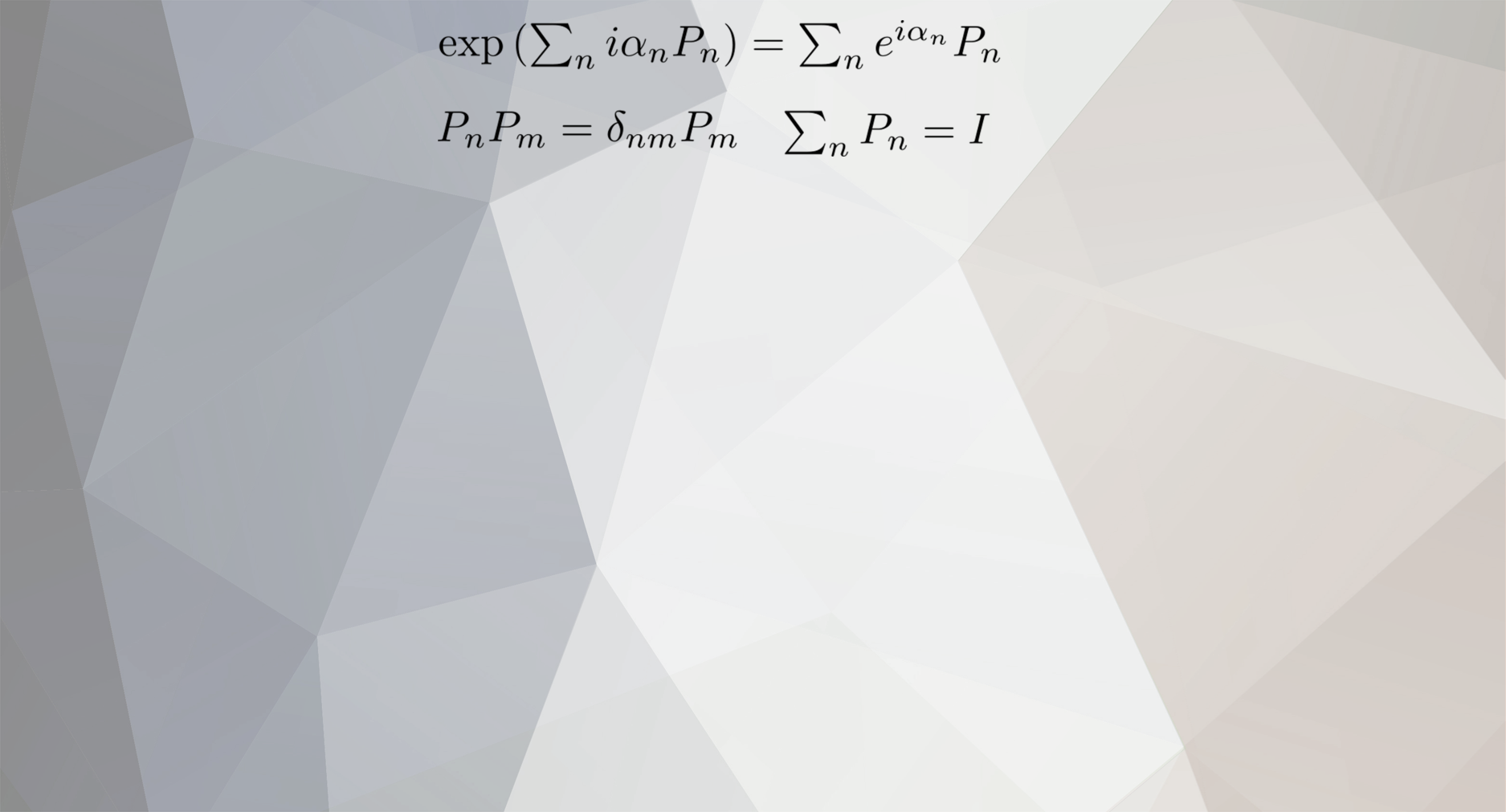
Everything posted by joigus
-
Is E=MC² the optimal description of nature?
Then it definitely isn't "the optimal description of Nature." The equation is also dimensionally inconsistent. Then you have an indetermination of the kind \( 0/0 \). It still has nothing to do with \( E = mc^2 \). Solving it is not difficult, but it makes no physical sense.
-
What are you listening to right now?
Nice video. Is that a Brooklyn accent?
-
Time is a bubble
The devil is in the details. Show us some, please.
-
What Ever Happened to Laika?
🤣 Happy Festivus.
-
To abstract or not to abstract
I don't think Zaps, or John, are trying to get you banned. Sounds to me that they're trying to corner you logically. That is always something most people of reason, always in our hearts, thank our intellectual opponents for --or should--, and deeply regret if we ever don't, and get carried away. It's actually the highest compliment you can get from people like them or like me. It's like having it said to you: "You are no idiot, I'm sure somewhere in there is a reasoning agent". I think you came closest to getting banned when you decided to drop reason and started applying names to other users (namely, myself and John, "fools"). You got it completely wrong. We'll never give up on you as long as you try to use reason and evidence, if I know what their motivation is. And I think I do. I'm sorry, but the Bible, although a beautiful at times, valuable always, document, is not a valid source of reason and/or evidence in general.
-
Cosmic Background Radiation and the Electromagnetic Field
You're absolutely right. I tried to convey that with, The energy dependence you're referring to would indeed be reflected in the \( \int d^{4}xe^{ipx} \) integrals, to which you'd apply the cutoff \( \Lambda \) for the given energy range. So, as you say, it'd be something like, \[e^{4}f\left(m/\varLambda\right)\] I was kinda fixing this temp-dependent factor, or normalising it to 1, if you will. At that energy, the \( e^2 \) terms would totally dominate the photon-photon ones. Low-energy photons are never seen to scatter off each other at room temperatures, let alone at cosmic-background energies.
-
What Ever Happened to Laika?
For whatever reason, a question that has been in my mind for many years, "what ever happened to Laika?", popped up again yesterday. Maybe it's because I tend to feel sad in Christmas. It's a recurring theme for me. Christmas seem to be all about loss. And I'm a Cold-War kid. In recent years I've learnt from paleo-anthropologists that we probably owe much of what we are to the presence of dogs in our lives, that goes back tens --if not hundreds-- of thousands of years at the very least. https://phys.org/news/2019-02-prehistoric-humans-dogs-death.html We owe them so much. Anyway, I found this story answering all my questions. It's not a happy story, I must warn you. https://www.smithsonianmag.com/smithsonian-institution/sad-story-laika-space-dog-and-her-one-way-trip-orbit-1-180968728/ I always wanted to know what ever happened to Laika. Merry Christmas
-
Cosmic Background Radiation and the Electromagnetic Field
The QED matrix element for this process "goes like", \[\textrm{Tr}\left(A_{\mu}e\overline{\psi}\gamma^{\mu}\psi A_{\nu}e\overline{\psi}\gamma^{\nu}\psi A_{\rho}e\overline{\psi}\gamma^{\rho}\psi A_{\sigma}e\overline{\psi}\gamma^{\sigma}\psi\right)=e^{4}\textrm{Tr}\left(\cdots\right)\] There are space-time integrals involved, but roughly speaking the probability for this process is controlled by the number, \[e^{4}\sim\left(\frac{1}{137}\right)^{2}\simeq5.328\times10^{-5}\] Which, again roughly speaking, means that this process of photon-photon scattering takes place 5 times every 100'000 times photons cross paths. The internal legs of the diagram represent two pairs of virtual e, e- pairs once appearing and once disappearing, and two processes of electron-photon and positron-photon scattering. At large enough scales what you see is photons scattering off each other every once in a blue moon.
-
Possible genetically different gametes?
Going backwards in the Punnett square, taking into account possible restrictions on the parental generation (wild types, homo-/hetero-zygotes, etc.) https://en.wikipedia.org/wiki/Punnett_square#Dihybrid_cross
-
Heaven - Rebirth as a Dog or Cat
Why do you write "Dog" with capital D? Is this some kind of palindromic play with words? Why can't you reincarnate into a bat, or an eland, or a moss? Does it have to be a domestic mammal?
-
Cosmic Background Radiation and the Electromagnetic Field
Sure it would in principle, those would be focalised beams of photons that would meet those completely thermal photons, here and there. But the chances of two photons scattering is completely negligible in QED at given temp. It is this process: https://en.wikipedia.org/wiki/Two-photon_physics
-
Cosmic Background Radiation and the Electromagnetic Field
You can treat the EM field classically; then there is a particular configuration that is consistent and is made up of an E that gives rise to a B, that gives rise to an E, etc. without any charge density sustaining it. Those are so-called "vacuum solutions" of the Maxwell equations and correspond to radiation, any EM radiation. The CMB is made up of these EM waves in the vacuum. They are clearly distinguishable from static fields. The quantum picture is one of photons, of course. The CMB is an EM field, but it's completely thermalised. So it's not like the light from a light bulb, or from the Sun, or from a diffraction grid, let alone from a laser. It has no particular direction, and has a considerable dispersion in frequencies. But most of the photons are around, \[\frac{\hbar\omega}{k_{B}T}\sim1\] in frequency \( \omega \) for \( T = 2.7 K \) of absolute temperature, where \( k_B \) is Bolzmann's constant. so it's very cold. You can shield almost perfectly against EM fields by using a conductor.
-
To abstract or not to abstract
Nobody said that. You don't want to learn science, that's all. Nobody said "any matter whatsoever". See how you are intellectually dishonest? Doesn't your Bible tell you not to bear false witness? You put words in other people's mouths. Now, that doesn't surprise me, really.
-
Is E=MC² the optimal description of nature?
Swansont's points well taken. Also: That's not an integral equation. There's extensive work in mathematics about how to solve integral equations. In what variable? Do you mean, \[ F\left(t\right)=\left[M+F\left(t\right)\int_{0}^{t}v\left(t'\right)dt'\right]\frac{v\left(t\right)}{t} \] as an equation for \( v \)? But \( F \) depends on position. What happens at \( t=0 \)?
-
Why are QM effects only found at sub-atomic levels?
Ferromagnetism,* superconductivity, and superfluidity are among quantum effects that can be seen with your own eyes. It is true that the context of QM par excellence is the very small, though. That's due to the smallness of the quantum of action when compared to ordinary experience. * https://en.wikipedia.org/wiki/Ferromagnetism#Explanation
-
Why does x^2 depend on 2 input values?
Agreed. And it's the bizarre nature of your questions. If you keep going on like this, you'll never get around to mathematics. I cannot emphasize enough how much attention you must pay to these tips: I could hardly agree more. If you're trying to climb Mt. Everest, and you're looking at another summit in the distance, you're gonna fall through a crack. Does that make sense? And for Pete's sake, solve a simple linear equation, get pleasure from it, and step on towards a more difficult problem. And keep going. Get something under your belt, however modest, ASAP. No matter how simple. Edit: Oh, and another thing. You're getting excellent advice here. Don't pay heed to "Daft Science" or "CrackpoGenius" or similars who might tell you how much more intelligent than others you are. They're distracting you and you've gone astray by their compliments before. I've seen it happen. That's another crack in Mt. Everest. Compliments are very distracting. Reliable information is gold.
-
What are you listening to right now?
- The two slit experiment ...a sensible answer
I did take a look at your channel, I don't obligate you to anything, and I do not have the power to ban you nor any will to do so. I agree with @studiot that it's a good example of a very bad simulation of science. If you care about these things, you should take time to learn them. Pressure does not require contact. We understand today that contact forces do not really exist, in the strict sense. It's all fields. You seem to ignore also that there are models of QM based on particles following the Hamilton-Jacobi equation with a quantum potential. They reproduce all the kinematical results. It's not that you don't understand pressure, or interactions monitored by fields, etc. It's, as very often happens in the realm of crackpottery, that you couldn't care less. You should open your ears and you could learn something in these forums from other users who know more than you. I have.- On Four Velocity and Four Momentum
"Makus Henke" is Markus Hanke, "Goigus" is Joigus, and as to \( v_{12t} \) nobody knows what it is. It can be nothing other than a typo, you clearly meant either \( v_{1t} \) or \( v_{2t} \) (otherwise you're summoning something here which is neither \( a_1 \), \( a_2 \), \( b_1 \) or \( b_2 \), the four numbers of your previous (correct) mathematical lemma. Once you either correct this typo, or tell us what \( v_{12t} \) is, I'm afraid nothing's going to save the incorrect proof, for reasons very much already explained. Anamitra Palit should reconsider his writing before Joigus (at least) considers his views any further.- The two slit experiment ...a sensible answer
You obviously have problems processing what you read, or do not read at all. Go back to my words in and around "if that's the case". The rest of your blabbering is not worth my time.- On Four Velocity and Four Momentum
No. There is a time component alpha and the corresponding Euclidean norm of the 3-vector alpha (that's why I wrote it in boldface). Same for beta. IOW, there are four numbers whose value I've chosen to be 1, producing a very clear counterexample of your assertion. It's very common notation in relativity. \[ \left( \alpha^{\mu} \right) = \left( \alpha, \boldsymbol{\alpha} \right) \] Do you understand the notation now? The question about constraints for physical 4-vectors, that Markus, Ghideon and I have told you about, still stands. As I said, Anamitra Palit must go back to elementary relativity books.- The two slit experiment ...a sensible answer
It's common courtesy, and how relevant it is remains to be seen. I also gave you the benefit of the doubt. So far you haven't used or mentioned any sensible physics, and you seem not to understand very basic principles (pressure, wavelength, action at a distance...) and deny evidence, so the doubt is vanishing fast.- The two slit experiment ...a sensible answer
In contradiction with experiments, then. Thanks for the heads up. Although I don't think living on welfare, if that's the case, automatically disqualifies you to do good science. Some folks may be able to keep a no-nonsense attitude and have a lot of time on their hands at the same time. I don't think that's impossible.- Political Humor
Duly noted. Is there a deadline to start my new funny self? Am I allowed to be funny in the sense?:- Did China's one-child policy save the climate?
Well, yes. Japan is perhaps a better example of need for restraint. But the topic was China. That's why I mentioned China. - The two slit experiment ...a sensible answer
Important Information
We have placed cookies on your device to help make this website better. You can adjust your cookie settings, otherwise we'll assume you're okay to continue.
