-
Posts
17639 -
Joined
-
Last visited
-
Days Won
93
Content Type
Profiles
Forums
Events
Everything posted by studiot
-
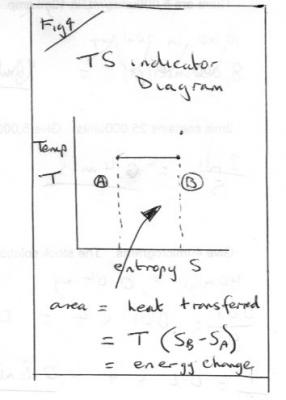
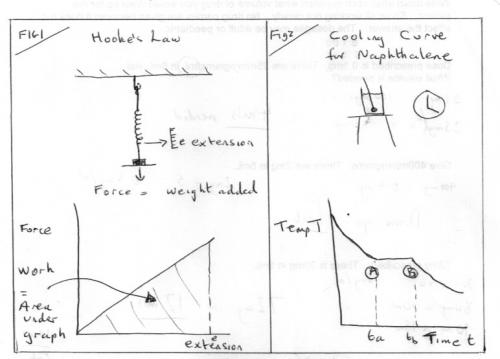
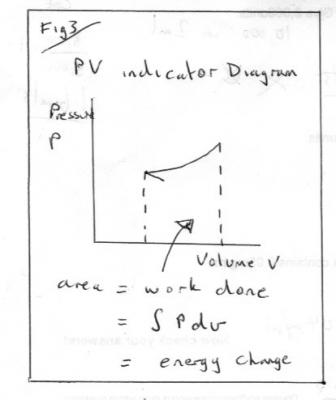
Entropy part 1 Entropy, like energy, is a derived quantity, not directly measurable. Today we have two quite different approaches entropy. It was one of the great triumphs of 19th century science to show these different approaches to be concerned with the same physical property, albeit from different viewpoints. Confusion often arises if and when inappropriate parts of one view are mixed with the other. Historically entropy was first introduced by the technologists and scientists interested in heat engines, particularly steam engines. These workers were concerned with plain down-to-earth matters that have real substance in out physical world that they could measure – We now call these observables. The second approach was abstract and theoretical and founded in the Kinetic Theory. Boltzman’s equation Entropy, S = k loge (W) is one of the great fundamental equations of Physics that has been expanded beyond energetics and thermodynamics to other fields such as information theory. This treatment is largely qualitative, for understanding, rather than quantitative for carrying out calculations. But it does involve some maths and underlying Physics. You should ask if I introduce anything you have not met before. To start with something familiar we have to go back to the time of Hooke and Newton and consider stretching and cooling as in the two diagrams Fig1 and Fig2 which have given rise to two modern day experiments for A level students. A great deal of Physics can be learned from both these two experiments that is not always put forward by the texts. They did not have a fully formed concept of energy, let alone entropy, in those days but they gave us the beginnings of modern mechanics (Hooke’s Law) and Thermodynamics (Newton’s Law of Cooling). In Hooke’s experiment we have two observables, the load or applied force and the extension. We can plot a force/extension graph and use it to find the energy input (= work done) as the area under the graph. Instead of getting hung up on the graph being a straight line two things to take from this 1) The use of observables to calculate a desired derived quantity. 2) The desired quantity (energy) is simply the area under the graph. This was the world’s first use of such a diagram . I will return to Fig2 later, but what do you think the observables are? Fast forward through the next hundred years or so, to the time of James Watt, during which Joule and Count Rumford put energy on a firm basis. In particular they showed that heat is a form of energy, a property of matter, not a special fluid that we add matter or take away from it. James Watt was concerned with steam engines and his directly observables were pressure, volume, temperature and mass. He was the first person to emulate Fig1 by drawing a pressure-volume graph and realising that the area under this graph also represents energy or work, as in Fig3. Clever mechanics soon created a mechanism to draw these PV diagrams, directly from the pressure gauges or indicators of the time. Such diagrams became known as ‘indicator diagrams’. Now this was all very well for pressure and volume, but what about temperature? So let us start by looking at Fig2 more closely. Newton’s cooling experiment (usually conducted with naphthalene today) is a temperature-time graph. It has three portions. Two decelerating curves where the temperature is falling according to Newton’s Law of Cooling. But in between these is a flat, horizontal, section during which the temperature does not fall at all. Yet heat is being lost all the time. This is our first inkling of entropy, and our first connection to the kinetic theory. Fast forward another hundred years whilst thermodynamics develops and the steam engineers want to link the other observable, temperature, to the energy input or outputs. So they look for a simple property that can be paired with temperature to produce a graph where the energy is the area under the graph, like the indicator diagrams of Hooke and Watt. Clausius came up with ‘entropy’ and it was given the symbol S. Entropy is nothing more than the property on one axis of an indicator diagram that yields energy as the area under the graph. They called this a T-S indicator diagram. Fig 4 Note that in order to create an indicator diagram using temperature as one variable they had to give up using only observables, but they set about producing tables of entropy for all conceivable situations and engineers of today use these to look up the entropy. So there you have it Energy = entropy times temperature Or Entropy = energy divided by temperature So it is measured in energy per degree (Kelvin). But in that century of development there were some twists. Many types of energy were identified. For entropy it is not any old energy but specifically heat energy. Mechanical energy is not included. So all the force times extension work under the Hooke graph in Fig1 is not included. In Fig 2 I have marked two points, A and B. Heat is being evolved but the temperature is not changing. The entropy change = Area under the TS diagram in Fig4 and I have marked A and B here as well. Today we have also identified many other pairs of variables that can be combined to form indicator diagrams. Once such pairing is surface energy and surface area, which is what started this thread. There are plenty of points to expand on here so I think that is enough for part 1.
-

Waveform of an electron
studiot replied to petrushka.googol's topic in Modern and Theoretical Physics
Perhaps you don't understand plain English. You clearly misinterpreted what I said because at no point in my life have I ever claimed that a free electron is an orbital, or even connected to one, even when I was at school. I actually asked a question because you claimed congruence between electrons and orbitals and I asked question 3 because I wanted you to demonstate this claimed congruence for a free electron. You cannot have it both ways. Either an electron is an orbital or it is not. Which are you claiming? You have been very fond of telling me that I'm wrong on this forum, yet I did not initially flatly contradict you. Rather I offered you the opportunity to think again by expressing doubts and requesting more details, indicating the areas of my doubts. Your response was a direct personal attack, which has continued in that vein since. -
A lump on one side of the washing machine huh? There is one place in a washing machine the washing stays away from. The axis of rotation. This represents a fundamental difference from the Earth's continents. Pangea was not the first super continent, nor is it likely to be the last. But look at today's globe. The continents are lumped together, not equatorially or on one side of a spinning globe, but spread around the axis concentrated close to the poles. http://www.nature.com/nature/journal/v482/n7384/abs/nature10800.html
-
OK I will start the ball rolling. First the word abscissa refers to plots of specific points on 2D planar graph paper. It is the coordinate relative one of the two sets of gridlines, by convention the horizontal gridlines on the graph paper. The other coordinates are called ordinates. By convention these are relative to the vertical gridlines on the graph paper. Now for the 'curvature' of spacetime. This depends upon your point of view. Here is an analogy, due to John Baez, a noted californian physicist. Two men stand at the equator and look directly at each other. They both mark off a right angle from the equator towards the North and start walking, both keeping straight on due North. That is as far as each can tell they are each walking in a straight line and they check this by checking the perpendicularity as they cross each parallel of latitude. As they approach the pole they realise that they are coming closer and closer together, although both are walking along a gridline perpendicular to their startline. So what is happening? Explanation 1) There is a mutual attraction, a sort of personal gravitational force drawing them together. Explanation 2) They are somehow not wallking in a straight line but a curved one. Now this example is not yet into spacetime, it is simply 3D space. But it is sufficient to explain quite a few things. The men are confined to a 2D world, the surface of the Earth. We call this a manifold. The manifold is simply all the places (points) accessible or available to the men. From the point of view of an outsider the surface (manifold) is curved in 3D so the gridlines of longitude converge. Further they are not straight but curved. So the outsider goes for explanation 2 From the point of view of the men, who cannot (let us say for the purpose of this exercise) see 3D, they cannot see this curvature So the men feel justified in going for explanation 1. There we have two explanations, a curved manifold or gravity in our model. But there is a further twist. The men have a choice. They could carry on with their coordinate system of lines of latitude and longitude, completely within the manifold. Or they could postulate the existence of curvature in a third dimension and draw up an X, Y, Z coordinate system. Then their paths become space curves (arc of circles) and they could call them geodesics. Projecting this forward to 4D spacetime we can do the same thing.
-

Waveform of an electron
studiot replied to petrushka.googol's topic in Modern and Theoretical Physics
Everyone deserves the right of reply. But not to call the other persons statement nonsense. Particularly when you specifically said "The orbital IS the waveform of the electron", emphasising the word 'is'. Now some electrons are free. So I asked you to describe the obital that is the electron, in this condition. -

Waveform of an electron
studiot replied to petrushka.googol's topic in Modern and Theoretical Physics
An empty orbital has more in common with an empty bookshelf. Whilst the book is on the shelf it is on a ledge in a potential energy field. If I remove the book does the shelf disappear? But as swansont has pointed out there is more to quantum orbitals than just energy levels so they are more complicated than bookshelves. -

Waveform of an electron
studiot replied to petrushka.googol's topic in Modern and Theoretical Physics
DrP, you should not imagine the comparison with your guitar string. Orbitals as solutions to the Schrodinger equation only bears a passing resemblence to the solution to classical wave equation which your cuitar string obeys. If you twang the string you can see a shape in space describing the vibrations. If you are inhumanly very very quick you can catch the string at the top of its vibration between your fingers. At this time ther is no possibility that the string will elude your grasp simply because it is at the opposite side of its vibration. That is what you see is a time averaged blur comprising mostly empty space. The orbital is not like that. Wherever you 'grab' it you will have some interaction with any electron in that orbital, as any atom approaching to bond will experience. The electron is not on one side of the vibration at one time and on the other side at another as the string is. -

Waveform of an electron
studiot replied to petrushka.googol's topic in Modern and Theoretical Physics
Wow, that is rather peremptory. Did I offend you? To answer your comments in reverse order 4) So you are saying that if I remove 1 electron from the 1s orbital of Helium, the orbital I took it from collapses? So what happens to the other electron in the 1s orbital? 3) So is this a claim that a 'free, unbound' electron no longer obeys quantum mechanics in general and the wave equation in particular? 2) So how do you explain bonding orbitals and anti-bonding orbitals? 1) So what? well this is tied in with question 4, already answered. -

Waveform of an electron
studiot replied to petrushka.googol's topic in Modern and Theoretical Physics
Not convinced. 1) An orbital can contain more than one electron, 2) What are you suggesting empty orbitals to be? 3) Please describe the 'orbital' of a free electron. 4) If an electron becomes free, ie leaves an orbital, what is left behind? Does the electron leave part of itself behind? -

no such thing as "infinity" in the real world (split)
studiot replied to cladking's topic in Speculations
The whole point of this thread appears to be that you wish to demonstrate the truth of its title. Unfortunately the title is imprecise since it employs imprecise words such as reality and existence. Existence in particular has a specific meaning in mathematics, which does not directly correlate with our ideas of reality, physical or otherwise. But you also asked a specific question of a mathematical nature, which seemed to me to question whether infinity could be handled mathematically and I gave a specific answer. My answer, as does your reply quoted above, showed that it most definitely can. So if you want to you can handle very large numbers or infinity mathematically. We are agreed. -

no such thing as "infinity" in the real world (split)
studiot replied to cladking's topic in Speculations
If a number is large but not transfinite it will follow the rules for finite numbers, however big it might be. So , for instance, given any integer, however large, I can easily make an even number from it (guaranteed). If the number is transfinite then you can follow one of the schemes of rules for the manipulation of such numbers. There is more than one available and they are all different from the schemes for the manipulation of finite numbers. -
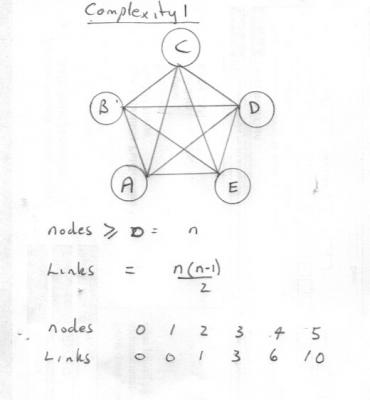
In order to put some flesh on the bones of my question here is an introduction to infinite and complexity for discussion Infinite We say an infinite something. But what? Consider the following two lists (sequences in maths) 1, 2, 3, 4.................. tan (0), tan (15), tan (30), tan (45), tan (60), tan (90) The first list is an infinite list of positive integers. That is is does not terminate or goes on forever. The list has no end. So we say it is an infinite list or sequence. But every one of the integers on that list has finite magnitude, ther are no infinite 'numbers' on the list. The second list terminates or contains a finite number of items. So it is not an infinite list. Yet one of the items on the list has infinite mgnitude. This is what I mean when I say that we have to be clear what we are applying the adjective infinite to when use it. As above some properties may be infinite, whilst some may remain finite. Complexity. This is a noun and again it can be taken in more than one way. Here is one interpretation, that can lead to a concept of infinite complexity, along the lines of the first example above ie it never ends. Consider a list of items, such as facts, theorems, properties etc. Call these items A, B, C, D, E etc Now these items may be linked by connections of some sort, eg equations or whatever. The totality of all the connections can be regarded as a measure of the 'complexity' of the system. The following diagram shows the maximum number of single links for 5 items, where every item is linked once to every other item by connections shown as lines. A formula and list for other numbers of items is also shown. You can see that in this model the system comprises the items and the links and the complexity is given by a simple formula such that the complexity remains finite so long as the number of items remains finite. However the complexity becomes infinite for an infinite number of items. Does this help the discussion?
-
I don't know, but I do know that I made a genuine offer to rationally discuss the terms you used, by asking my question in post#25 to which you have not responded. It is against the rules of this forum to ignore polite questions in this manner.
-
Whilst you deserve acknowledgment for adding the important word 'might', you still have not explained what you mean by complexity. This is important because infinite is an adjective that has at least a couple of meanings, but it has to have something understood to be applied to. So what is your explanation of complexity and what about this explanation can the word infinite be correctly applied to?
-

Converting chemical structure to a molecular formula
studiot replied to Aussiek's topic in Organic Chemistry
This example brings up another point for down the track. The pentagon is a closed shape, called a ring or cyclic structure for obvious reasons, however it is not the most common or important one. You should always be aware which ring you mean, it is particularly, if your glance is too quick you can confuse the pentagon and hexagon. The hexagonal benzene ring is the most common arrangement and the basis for a large branch of organic chemistry, the aromatic compounds. http://www.2b1stconsulting.com/benzene/ -
Well we should be well protected since all our mods are now godesses.
-
A walks towards B. But B is walking towards C who is walking towards....... etc So A is constantly turning inwards as he is always walking directly towards B. Draw a sketch and you will see what i mean.
-
Foxy has a point. This reminds me of the following question. Four circus performing fleas are placed at each corner of a 1 inch square ABCD. At the ringmaster's whisltle all the fleas start to walk towards their neighbour. So A walks towards B, who walks towards C who walks towards D, who walks towards A. They all walk straight towards their target at all times. Since they are all in motion each flea walks a spiral path and they meet in the centre. How far does each flea walk?
-
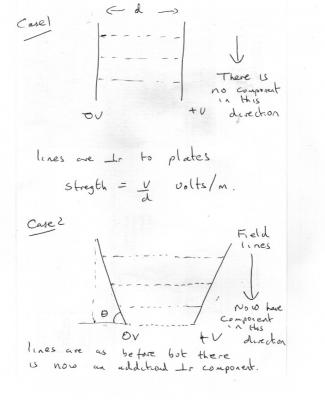
In all cases the field strength equal the voltage divided by the plate separation E = V/d But in the second case the plate separation varies, due to the geometry so this induces a second component, perpendicular to the original, as the field strength increases from bottom to top, as in the diagram. This causes the electron's path to curve. Is this really an A level question?
-
1) What do you think the path of the electron will be in both cases? 2) sketch the lines of constant field strength in both cases. 3) What is the angle between the constant field strength contours and the Force on the electron. 4) What do you think this does to the path of the electron?
-
Thank you. A single hydrogen atom has one electron. So this is the only case in which the issue of which electron does not arise. swansont's replies show that even here the possibilities are beginning to get complicated, and I am struggling a little bit to find a level to answer. So here goes, here is the simplest answer I can think of. Moving the electron to a higher energy level moves it further out from the attracting positive nucleus. So electrostatic attraction will pull it back in, if at all possible. This is similar to saying that if you throw a stone up, gravity will pull it back down to Earth. Also similar to gravity, where if you (or the HULK) throws it up hard enough the stone can leave the Earth entirely, it is possible to remove that electron and take it away somewhere so that it will never fall back o its original state. That is what I meant by the environment and the process of excitation. Not important in single hydrogen atoms, but very important in thermionic emission or conduction bands in semiconductors or conductors.
-
Several things need amplification here. First and foremost the energy state belongs to the environment of the electron, not to the electron itself. Secondly the above quickly glosses over the means by which the excitation occurs. Thirdly it may not be that specific electron that 'falls back' to the lower state. So please tell us more about the context of the question. Is the electron in an atom, a molecule, a metal, free space or what before excitation? Secondly what method of promotion are we discussing, light, heat, electric field or what?
-
The commonly called Principle of Equivalence boild down to an assertion that the m in inertial equations, such as Newtons 2nd Law, is the same as the m in gravitational laws (Newtons included). This has been repeated demonstrated experimentally to ever greater levels of precision. Eotvos experiments confirmed it in Einstein's day. The principle has often been quoted as its logical consequence or deduction viz that a blindfolded observer cannot tell the source of a hidden acceleration she experiences.
-
Do electrons collide?
-
Fine, look out for the next post about entropy and surface energy. BTW what are you studying? You originally said this came up in Chemistry, so I have been trying to put things from a Chemistry point of view.