-
Posts
17639 -
Joined
-
Last visited
-
Days Won
93
Content Type
Profiles
Forums
Events
Everything posted by studiot
-
No talking to you isn't all that bad, in fact you seem to be progressing towards proper discussion. I try very hard to read and address the contents of the posts of others inlcuding yourself. But when I asked for your comments on carbon dioxide in the atmosphere, you responded by talking about solid carbon dioxide. Which I hope you will agree is frustratingly irrelevant. But this last time you seem to be actually responding to what I wrote, so let us start again, without preconceptions. I think you seek a mechanism to explain how EM waves can pass by, or be absorbed by, or reflected by objects in their path. Ideally this mechanism should offer the conditions when each possibility will happen and concur with the results of real world observations. Please confirm that this is the case because I am trying to explain this to you. I also said that the easist explanation for this is wave theory. By this I meant classical wave theory, which in my opinion is sufficient to discuss the matter. My starting premises, based on real world observations, are: The structures of aerials for TV and radio broadcasts allow the broadcast waves to pass by and are sometimes reflected by these structures, but only extract the energy (ie absob at least some waves) for certain specific wavelengths which they are said to be tuned to. For simplicity I will take the simplest possible aerial, a length of conductor such as a straight aluminium or copper tube or wire. It is an observational fact that the wavelengths this aerial is tuned to depend upon its length in much the same way as and organ pipe can be tuned to a particular sound wavelength. Please confirm that you consider there observational facts. I am saying it like this because you chose to simply quibble by ignoring various helpful comments I made to enliven and enrich the discussion. I propose the development and extension of this theory to atmospheric carbon dioxide molecules. Working together this can be achieved. Continuing to work in seoparate compartments, it is unlikely the discussion can progress. The choice is yours.
-
J.C.MacSwell Since the OP hasn't thanked you for such a clear and complete answer I will. +1
-
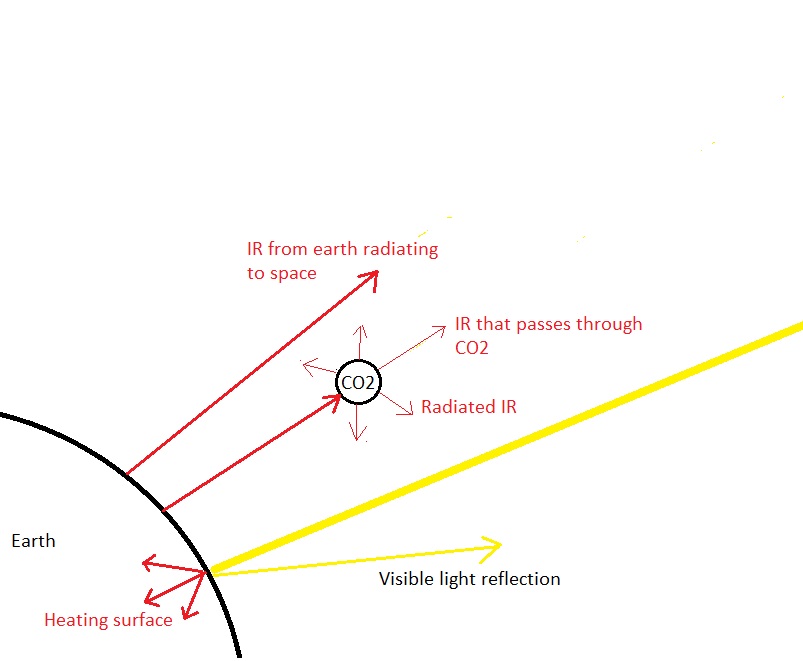
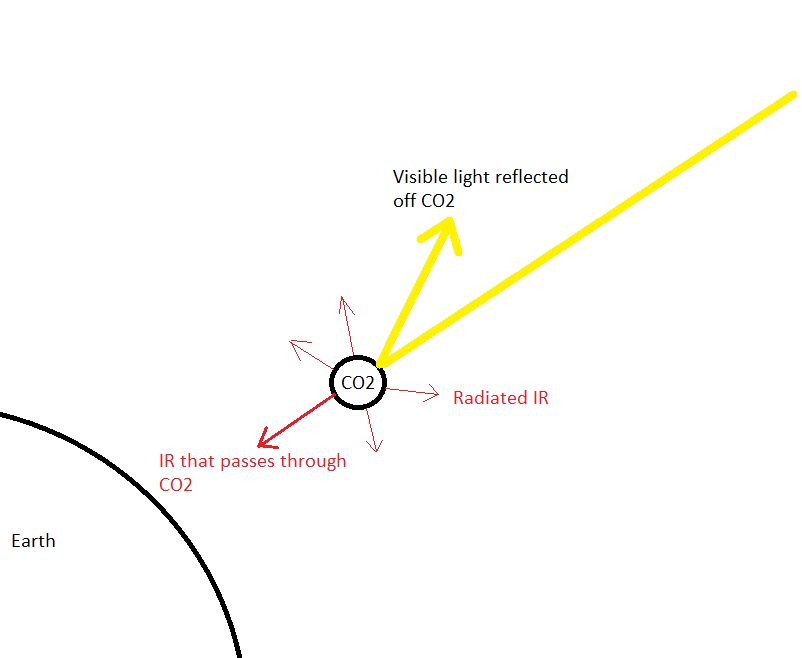
Despite your unappreciative/unhelpful remarks in your last reply to me I will try one more time to help. The reason is best found in the wave theory of light and is the same reason we cannot see atoms or electrons with a light microscope. The wavelength of visible light is just too long by several orders of magnitude to resolve distance this small. The radius of an electron is of the order of 10-16 metres The radius of a carbon or oxygen atom is of the order of 10-10 metres The wavelength of visible light is of the order of 10-7 metres Since you like sound analogies, it is the same reason low frequency sound can diffract round objects, whilst higher frequencies sounds are blocked. In your sketch you have shown the wave being much smaller than the CO2 molecule. In fact it is the other way round. A visible light wave is ten thousand times larger.
-
This is where the engineering decisions and trial calculations come in. Yes the water could be pressurised to raise the temperature, or the transfer fluid could be steam or oil or a commercial high temperature 'refrigerant' such as you find in heat pumps. Yes a suitable piping loop could evaporate 3500 litres, but what is the recharge time? That would determine if it was fast enough.
-
Come on, are we talking sense or not? I asked why we could see through a carbon dioxide atmosphere, either as dilute as the Earth's atmosphere or pure, if carbon dioxide reflects visible light. Your answer apppears to be that we can't see a suspension of water droplets (clouds or fog) which is true but irrelevant. One of the important characteristics of wave motion is that reflection occurs at a boundary. The boundary is not at the atomic scale but water doplets provide an ideal boundary to disperse the light rays by reflection. I didn't notice much solid carbon dioxide around when I got up this morning either, so what is the relevance of the transparancy or opacity of the stuff?
-
Good morning, James. It looks as though our timezones clash but never mind we will get there. Yes lots of very useful fill-in information. Thanks. No you are not an idiot and you have done exactly the right thing by asking. In fact is is a real pleasure to have a pleasant, intelligent and productive conversation rather than a dispute with some troll. OK let's get the temperature thing cleared up first then see what practical advice can be offered. Temperature is not a measure of the heat quantity in a body, it just tells you which way heat will flow naturally. There is a great deal of heat in the ocean, but not easily accessible because the temperature is low. On the other hand, the temperature of a lighted candle is high, but the heat content is low so you don't burn yourself if you extinguish it by pinching between your fingers. There were two reasons for my question aboyt the exhaust gases. I hope you understood the first one about need to leave some energy for the exit kinetic energy of the gas. As a materials specialist I assume you have covered chemical energetics. The second reason is to do with the safety aspect. Fluing requirements (in the UK at least) are very specific to prevent noxious gases from entering the living environment. There is a substantial minimum flue cross sectional area (aka pipe size) which would not fit well into your tank. Take the cover off a domestic or commercial boiler and look. Further, in order to maximise heat transfer you would need thin walled, thermally conductive duct material. This would contain hot corrosive gases inside and be surrounded by hot water outside and thus difficult to avoid corrosive heat damage. A better arrangement from both the safety and practical point of view might be an indirect heat exchanger system. Piped water (or other fluid) from a small reservoir is heated within the exhaust duct or by being wrapped around it. This water is then passed through the water in an evaporator, rather than a tank, inside sealed heat exchanger piping where it heats the water you want evaporated before returning to the small reservoir. Because the heat exchanger fluid is sealed it can be clean and neither corrode the pipes nor deposit scale within them, preventing degradation of the system. The evaporator basin itself should be as spread out as possible, allowing maximum surface area to the evaporating water. It may be beneficial to blow the water vapour away with a fan to assist speed up evaporation. Electric heating elements can also be deployed in the evaporator to assist if there is not enough energy in the exhaust gas or you are just not running the burner. Since you presumably wish to recover the solids this would be easier from a flat spread out tray than from a tank. Cleaning such a tank is much easier. You can get an idea how much heat might be available by multiplying the calorific value of the fuel gas by the usage rate of the burner. Both will be reasily availble from the manufacturers and should ideally be stated on the equipment. That is the total heat produced as a time rate, ie per second or per minute. Remember power = rate of doing work = energy per second. If your system cooled the exhaust from 400o to say 150o, then the heat available to your evaporator will be up to (400 - 150) * Cexhaust where Cexhaust is the specific heat of the exhaust gas and will be readily available from tables. You do not want to go much below 150o as the one of the exhaust gases will be water, which you want to keep as steam for exhaust purposes. How are we doing?
-
Then why can I see through it? Even a high pressure tank of CO2 is transparent (apart from the tank itself) For most CO2 molecules the preponderance of all directions is either back to Earth or towards another CO2 molecule above it or to the side of it. I don't think most is lost to space, since most of the CO2 (a heavier gas than air) is beneath the upper atmosphere. Does glass reflect IR? Are you not getting mixed up between reflection and absorbtion? Google has lots of IR absorbtion curves for glass. Of course there is always a question of angle of incidence when it comes to reflection. Greenhouses receive insolation overhead, but the ground radiation averages a flatter incidence.
-
No I was kind enough to ask the same question in three different ways in an attempt to help you understand, since you seem to be having trouble understanding it. Thank you for that clear, if totally false, statement that "Light is electrons" The plain fact is that whilst electrons can in some circumstances emit light, yet in other circumstance remain quite happily dark (without emitting light), there are other sources of light generation besides electrons. Further by your theory, if an electron absorbs some light, one would expect to get more electrons if light were indeed electrons. yet if an electrons or electrons absorb light you have the same number of electrons as before. So it is up to you to explain these facts in terms of your hypothesis
-
You did not answer my question. Period. Since you made several statements to form your chain of reasoning, I am taking them one at a time and correcting them. You cannot expect the rest of the chain to be correct if the first statement in the chain is lacking. So please reply to my question. Without using existing light to generate heat, what is the connection between thermally generated light and electrons?
-
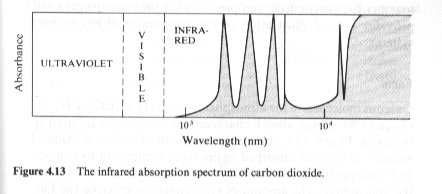
Here is the absorption spectrum of Carbon Dioxide. As you can see it does not absorb frequencies higher than the mid infra red. Why should it reflect anything it is not dense enough, even if the individual molecules could do so. (It is only .04% of the atmosphere) Note that water and glass are much denser and also transparent to ordinary light, but also absorb in the infra red. That is why they make good greenhouses too. Note most transparent plastics do not show the same level of IR absorbtion and make correspondingly poorer greenhouses. The CO2 molecules absorb the IR radiation from Earth, but excited molecules soon drop back to lower states, re-emitting that radiation. They do not reflect it back to Earth.
-
Yes, but the point is the phenomenon is empirical evidence to support my hypothesis. The Bremsstrahlung effect is an example of where high energy electrons produce x radiation (electromagnetic energy) as they are diffused by coming in close proximity to powerful electric fields in high proton count nuclei . In other words electrons in, EMR out Your reply has nothing to do with the fact that your original claim goes too far or even with the instances where it is factually correct. Your original claim was simply incorrect. So correct it and move on, because there are further significant issues with your hypothesis. I would agree that many sources of light are associated with electron activity, but how do you explain thermally generated light emitted by a hot body?
-
Can you try to narrow the focus of your question? You did originally ask about incident solar radiation the Earth's longer IR radiation in relation to Carbon Dioxide.
-
Note the first three lines of this reference. If you said sometimes the frequency is the same, sometimes it is different I could agree with you. But you chose to shout the all embracing "IN ALL EXAMPLES", despite your 'formal education in chemistry. At least you had the courtesy to reply me this time, unlike in the science & religion thread earlier today.
-
Just to reinforce this. Sound waves affect molecules considered as point particles acting on the entire mass of the molecules as one to creating pressure waves (the sound). The upper limit of such activity is a couple of hundred kilohertz in normal circumstances. EM radiation affects the relationship between the individual parts of the molecule (ie its constituent atoms). The best way to imagine this (It's too late tonight for me to draw the vibrational modes of molecules, you will have to look them up) is to imagine the (much lighter) atoms as balls with mass connected by springs. As such they perform various dances known as waggling, twisting, stretching and so on. Edit there are also modes of absorbtion and emission due to interaction with the subatomic parts of molecules, particularly the electrons for the present purposes. The frequencies of these motions are 10 to 100 gigahertz for IR and 100 to a few thousand gighertz for visible light. Many order of magnitude different from the sound vibrations.
-
Hello James, and welcome to Science Forums. Yes this is an engineering question, and I suppose you are an amateur engineer from your description of the problem. I am a little worried about the safety aspects of handling an exhaust gas at 400oC so perhaps you would explain in more detail what exactly you are trying to achieve. Such devices have been made, but the devil is in the detail so Do you really want to boil the water or evaporate it? Is this clean water or does it contain chemicals or other material that would need residue removal? What do you want to happen to the evaporated water and is it being replenished as it evaporates?? You have said 3500 litres of water, but given no indication of the quantity or nature of of the exhaust gases. (You do realise that you can't extract all the energy from the gas as it need some to clear the flue? Some manufacturers of condensing boilers have been embarrassed by this and other oversights)
-

Another hijack from Reconciling science and religion
studiot replied to Anonymous Participant's topic in Speculations
I don't know if you saw my previous post in the recent flurry but here is a true story I was looking at yesterday. In my part of the world it is hilly and often rainy. A man refurbishing a cottage wanted fancy oak faced flooring in the hallway. That is a deliberate design by an intelligence (I'm not saying great or small intelligence, just some intelligence) Unfortunately he also wanted fancy entrance doors flush with the outside. Yesterday it rained heavily and the water gushed down the hill behind the entrance, flooding into the hall and damaging all that expensive flooring beyond repair. Please comment on the intelligence of that design.