

UC
-
Posts
547 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by UC
-
-
1) managed to reset my alarm clock, but a fire alarm at precisely the minute it was supposed to be set to woke me up and kept me from missing an important exam.
2) assurance that I won't do too badly in my electronics course, even though I keep doing abominably on the tests. Yay professor's discretion.
3) short extension on my chemistry research-related reports, so my workload for today is physically possible.
4) 1 day of classes left, with no real work in either of them.
5) accepted for a research position this summer at a local college so I can live at home and spend time with my girlfriend. The other option was to be stuck doing research at school for 10 of 16 weeks during the summer working 9-5, 5 days a week and driving 4 hours home every friday night and 4 hours back every sunday night just to see her for a little while.
0 -
Well, temperature is the macroscopic realization of the kinetic energy of molecules.
I'm fairly sure that the answer you want involves the sharp and well-defined point where semi-free motion of the water molecules (water) is a lower energy state than vibrating rigidly in position (ice), but I don't know enough about this topic to say much. Ask a physicial chemist.
0 -
I'm currently working on a project to make a small scale car, potentially Remote Controlled, that runs off Hydrogen that I produce by electrolysis ON THE CAR
That's like saying "I'm going to eat this full course dinner so I have enough energy to run several miles to a McDonalds to buy a hamburger."
It would be vastly more efficient to just use an electric motor, not to mention lighter in weight and far less complex.
0 -
I think you can forget them in this case, they get deprotonated to the carboxylate ion which is negatively charged and so it's not attacked by the hydride.
Esters, IIRC are reduced by LiAlH4
Carboxylic acids are reduced to the alcohols as well, but one equivalent of hydride will be wasted deprotonating the free acid. For example, http://www.orgsyn.org/orgsyn/prep.asp?prep=cv8p0434
0 -
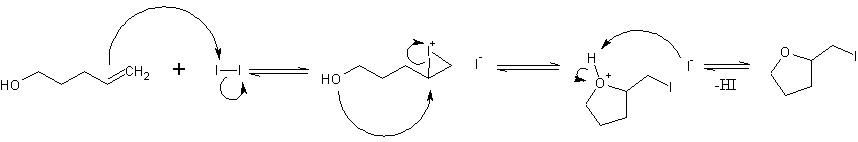
Iodine is very poor at adding to double bonds, so perhaps the first two stages normally equilibriate, but don't proceed to full addition across the double bond. I'm just guessing at that, but that's probably why iodine is used instead of other halogens, which would readily add completely across the bond forming vicinal dihalides.
Iodolactonization is also known, where the alcohol group is replaced by a carboxylic acid. The iodine sources I'm seeing are N-iodosuccinimide, I2, or a mix of NaI and FeCl3 with solvents like acetonitrile and chloroform at 0C
I don't know how well this reaction would work intermolecularly, but my guess would be poorly.
1 -
Not quite... you see, the hydrogen chloride you refer to is hydrochloric acid. It all has to do with solubility, as if the substances in question are polar, they will be soluble in polar solvents. If they are nonpolar they will be soluble in nonpolar solvents. As such, you need to account for the solubility (through Ksp) as if the substance is soluble, you will lose a certain amount of your existent yield. Thus, find out whether water is polar, and then in contrast, examine your solutes. You need to also see if there are any chemical interactions, as these can also deter your final yield.
No, technically HCl (g) is not hydrochloric acid. Hydrochloric acid refers to HCl (aq), although the term is often stretched to cover solutions of HCl in other solvents.
Ksp is for ionic solids. There is a solubility constant for non-ionic solids as well, but it's not Ksp.
Theophrastus- please check the date on a post before responding to it. The last post was 3 years and a month ago. I seriously doubt that Derrell is still doing this homework.

For anyone who cares anyway, as was suggested by the other posters, look at what kind of substances HCl, NH3, and H2O are. Like dissolves like. You may also want to search for "MSDS HCl gas" or similar and check the water solubility under the physical data section, to give yourself an actual number.
0 -
You can always buy it off of unitednuclear.com XD
Would it work to put some metal like Mg in NaOH solution? Granted, if it did work it would react with the water but I'm just curious.
No. Please learn what this means and how to use it: http://openlearn.open.ac.uk/file.php/3258/T357_1_ie001i.jpg
"Galvanic series" or "electromotive series" are helpful things you might punch into google, which I will not do for you.
Granted, everything is an equilibrium, so transiently and randomly, some magnesium might make a few sodium ions into sodium metal, but they will immediately give up those electrons to water or back to the magnesium ions that were formed.
0 -
the sun contains only a few of the lightest elements, mostly hydrogen helium and lithium, not much else. So no... although people thought that helium existed only in the sun for a while but it was just that it hadnt been discovered here on earth because it's so unreactive.
It's not so much that it's so unreactive, as argon was discovered in the atmosphere, but the fact that there isn't much in the way of sources for it. As far as I know, all of our helium is from natural gas sources. The helium is the result of alpha-decay of thorium and uranium minerals (the alpha particles slow down, capture some electrons and become [ce] ^4He [/ce]). It becomes trapped in the same impermeable pockets of rock as the natural gas.
I believe a uranium mineral that had the gas trapped in it directly was responsible for the original discovery on earth though.
1 -
3% H2O2 and slightly rusted (the rust seems to be catalytic) iron turnings, IIRC will get hot enough to boil/produce steam for a good long time
15% H2O2 with activated charcoal "bubbles" a whole lot (oxygen) and probably will eventually get hot enough to boil the water involved. The decomposition is fairly slow when I used the big chunks of carbon meant for fish tank filters.
If you mean "how can I make what looks like a plain glass of water boil for a long time," I have no idea if I can help you. You could aways pull a vacuum on it and it will really boil as long as you keep pumping out the water vapor
0 -
Yes, this is a simple metathesis/precipitation reaction.
Keep in mind that barium compounds are quite toxic and the hydroxide is very sensitive to reaction with [ce] CO2 [/ce].
0 -
What's a Ksp?
I'm afraid I've never heard of this, but possibly you use a different abbreviation than me.
It always helps to ask a clear question. We are maybe experts here, but if you explain to us all that you know, then we can better explain you what we know.
It's the solubility product equilibrium constant. It sounds to me like sjlopez is in analytical chemistry or at least a course that touches on some analytical concepts.
Can you please explain the experiment better. The calculations are probably trivial, but I'm not sure what you did with NaOH or the HCl.
0 -
The above also applies to the other alkali metals
Ohh, ohh, I think I electrolysed myself some cesium!Hermann is way ahead of you
 0
0 -
No, UC, you're absolutely rightr, as Ksp is the product of the molar concentrations of the dissolved ions. It's also useful as it governs, the common ion effect, which governs a change in the solubility equilibrium of a solution, in the case of the addition of another substance.
Nono, I meant about the alpha value. Usually if the question cares, it will give you the pH of the solution and Ka1 and Ka2 of carbonic acid.
0 -
[ce] Ksp=[Mn^2^+][CO3^2^-] [/ce].
where [] indicate concentration (molarity). Be sure to apply an alpha value for the fraction of carbonate that stays as carbonate. Keep in mind that it's 6am and I've yet to sleep, so I may be wrong.
0 -
Ditto for Magnesium, which keeps turning up as well, unless of course, you have sodium to waste.
0 -
The most oxidized product of glycerol would be something like dicarboxy-formaldehyde, but I can't find anything about it, and I wouldn't be surprised if it is not stable and will instead form formaldehyde and 2 CO2.
My first guess here is that glycerol doesn't want to oxidize any further without falling apart.
http://en.wikipedia.org/wiki/Mesoxalic_acid
less oxidized products include dihydroxyacetone (sunless tanning products) and glyceraldehyde (important compound of you're stereoisomer-savvy)
but yes, both are irrelevant to the discussion. I think glycerol would be more far more valuable as a feedstock than as a fuel.
0 -
not what I meant. I meant if you turned all the hydroxy groups into carboxylic acid groups, what would you have? it's basically citric acid with one hydroxy group removed.
That would involve magically adding carbons
 2
2 -
Agreed, all it does, is induce the corrosion of the magnesium metal, and release hydrogen gas, in the process.
Actually, I think the corrosion of the magnesium would be hindered by formation of a hydroxide skin. Unlike aluminum hydroxide/oxide, which you are probably thinking of, magnesium hydroxide is not amphoteric.
0 -
i see. Well I don't have any tin compounds, only tin... I imagine some SnO2 wouldn't be expensive, though, and tin's melting point is a LOT lower.
Can you make bronze by using a mixed ore?
I'm under the impression that tin doesn't reduce too cleanly, but perhaps I am wrong. Stannous oxide would be much easier to work with than stannic as well. The SnO2 of industry is a hard, calcined, largely unreactive compound. Molten alkaline fusion may be able to dissolve it as stannates though. PbO should be easy to get and use. Plus, you're making what, a couple grams of waste?
0 -
The syntheses of ethanolamine, diethanolamine and triethanolamine among other things relies on the reaction of an epoxide with ammonia or an amine.
I stand corrected.
0 -
2Mg + 2NaOH -> 2MgO + 2Na + H2
Aqueous solution of NaOH or something else?
not at all, especially in aqueous solution.
look up reactivity series.
I dont think sodium can be distilled out of the mix of molten NaOH and Mg too easily or you could cheat with Le Chatlier's principle and a lot of heat.
0 -
Where did you attain the hydrogen ion? In the electrolysis of pure (molten!)magnesium sulfate, you attain magnesium at one end, and a sulfate ion on the other. The sulfate then decomposes to form sulfur dioxide (a rather poisonous gas) and diatomic oxygen. However, while early on, many people see electrolysis as the source of all their problems, electrolysis of salts is often very difficult due to the high temperatures involved (as they must be in a liquid form), and use of an aqueous solution often interferes with the attainment of desired products. Without a high current, its also a rather slow process, certainly not an optimum one. It's not, by any means the "magic bullet."
MgSO4 decomposes before it melts.
0 -
Well, an amide certainly won't be basic enough, since they're roughly neutral. I have no idea about amines, but the link isn't working for me. I suspect that they are too weak, but perhaps under certain conditions.
0 -
Just a question about fluoridated anti-cavity mouthwash. The instructions say to swish for 1 min, not to swallow any, and not eat or drink for a period of about 30 mins afterward. They don't say whether you should or shouldn't rinse your mouth out with water after using mouthwash. Is this a sensible precaution to avoid swallowing some, or does it largely nullify the protection due to its not having the 30 mins sitting on your teeth?
I think it may have more to do with allowing the new layer of fluoroapatite set properly, in which case, rinsing your mouth out is not advised. i suspect a quick rinse with plain water is probably fine though, just not soda or fruit juice or something. It is probably quite possible to stain it at this point.
0

Concentrating Hydrogen Peroxide
in Experiments
Posted
AFAIK, it's not a true "explosive" so much as it vigorously and violently decomposes when catalyzed. If you were to put 90% H2O2 in an erlenmeyer containing even traces of copper, silver, manganese, etc. ions, you would readily make a rocket engine of several hundred degree steam and oxygen gas. chances are the erlenmeyer would shatter and scalding steam, peroxide, and glass would go be ejected in all directions, such as into your face.
I don't believe you're working in a school lab at all. I think this is BS to cover your arse.
High concentration H2O2 is made by reduced-pressure fractional distillation in dedicated glass apparatus, IIRC.