

UC
-
Posts
547 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by UC
-
-
Science, unlike religion, is a logical system. A system has a framework of basic information that is assumed to be correct (in this case, all sorts of equations that can't be derived from other equations). From these basics, it derives all other content (many equations in physics can be derived if you have enough time on your hands). A system has the power to accurately predict and explain based on it's contents. It is far from a perfect system, which would be complete, have no internal contradictions, and establish the validity of all assumptions (including the validity of deductive reasoning, but that's largely *the* big project for logicians).
Religion on the other hand is more like a list of "facts." If I have a question, I consult the large holy book of your choice and look up the answer. If the answer isn't there, I'm screwed.
0 -
Whoops, I meant to say aqueous ammonia not anhydrous ammonia

Wait, since some of you suggested in using pipette, would marking the pipette with calibrated mL lines work for titration so I could determine just how much titrant I used?
I've seen many varieties of burettes but the cheaper ones seems to be made of acrylic. About $19 to $25 USD without shipping.
I am eventually planning on buying a glass burette but since it will be much later, I thought that I need a substitute while that time comes.
How will you accurately calibrate a pipette without another method of measuring....You could buy maybe a 5ml mohr pipette (and pipette bulb), but they're rather crude and probably not much cheaper than if you catch a deal on a burette.
My burette is kimax glass with teflon stopcock. $20 on ebay...you just need to have patience sometimes and don't go by ebay store prices.
 0
0 -
Their were many theories that where illogical. Just look at radio astronomy.
Just look at Einstein's theory, he had to crawl through swamps to prove his theory.
The point is that evidence cannot pile up against my idea when their is no evidence for my theory. You can say whatever you want, you cant disprove it, nor can I prove it.
I mean, I cant set up a billion dollar experiment, like the real scientists do.
But their is a high probability, of this particle of existing. I mean just look around, their is enough proof for you.

Try taking an english course while you're at it. It should read something like this:
There are many theories that were considered illogical such as radio astronomy.Take Einstein's theories. He had to crawl through swamps to prove them.
The point I am trying to make is that evidence cannot pile up against my idea when there is no evidence for my theory. You can say whatever you want, but you cant disprove it, and I am a big fat smelly turd.
I don't have the resources to set up a billion dollar experiment, like the real scientists do.
But there is a high probability, of this particle existing. Just look around; there should be enough proof for you.

By the way, some lovely fallacies you have in there. You said earlier in the thread that there is a possible test for your idea (NOT A THEORY) Unlike other topics, such as the existence of a god, this means that we can in fact have evidence against it. You don't need to have any proof for your idea for others to point out errors and evidence to the contrary.
0 -
I'm not accusing you of a lie, I'm just being skeptical, as I should be in science.
In which case, allow me to accuse him of blatantly lying.
 0
0 -
Forgive me if this is a common knowledge question, but how does one go about producing iron, copper and/or alluminum sulfide... Alluminum sulfide is said to be produced from "ignition of the elements" and iron by "reacting iron and sulfur"?... Copper sulfide seems easy enough as I have copper powder:-) but can I use molten aluminum/ iron and sulfur or does it have to be powder? Any help would be appreciated. Thanks in advance!
The reaction of iron powder and sulfur powder is violent, as is the reaction of aluminum with sulfur (difficult to get to ignite and it reeks immediately after making it from toxic H2S). Copper sulfide needs to be made by adding a source of sulfide anion to an aqueous solution of a copper salt.
Treat them like pyrotechnics.
0 -
You misunderstand what electricity is fundamentally and how changing magnetic fields induce a current in wires (how a generator works). No electrons ever leave anything. Current flow is just electrons moving around in a definite direction in a loop of wire (okay, so it doesnt have to be in wire, but lets leave that out of the picture for now). These electrons are already there, but don't move in a definite direction in any old piece of metal. When pushed in one direction, they dissipate potential energy (voltage) across a load (something with a resistance; a lightbulb in this case) and it emerges as photons of heat and light. The net effect is that the energy you expend turning the crank gets turned inefficiently into light.
0 -
Any of the valve metals (tantalum, titanium, niobium, tunsten, hafnium, etc.) or platinum group metals (commonly platinum and nickel)
0 -
(except for blayzovich)
This.
thread.
is.
 0
0 -
Because current flow has nothing to do with more electrons, just the motion of the ones that are already there.
0 -
I'm hoping he means aqueous ammonia...
try ebay. You can get a burette for like $20. Or get a good quality graduated syringe. The latter won't really save you much money and the accuracy will suffer.
0 -
Use foam-core board
 It's a good deal thicker than what you want, but very lightweight and rigid.
It's a good deal thicker than what you want, but very lightweight and rigid.I've done two maglev track projects before (The "car" was constructed out of this material) and the stuff is more than light and strong enough to be held up by ordinary permanent magnets.
0 -
dont u all think that corrosiveness cant be the scale of strength as it also depends on the surface on which acid is acting. dissociation constant of HCl >HF still HF can Corrode glass. also just for the fact wikipedia lists HeH+ as the strongest acid in terms of PKa values(-62).
That particular power of HF is all about fluoride and it's affinity for silicon. Since all the acids do different things to different substrates, I don't think it's really fair to pick based on reactions of the anion. The only fair grounds to compare acids is the acidity and the acidity alone (what you are saying).
The pKa for HeH+ is entirely theoretical, by the way. There is no way that compound could ever be isolated and used in a lab. (perhaps a few molecules in some fancy physics equipment at most)
0 -
This is a guess, but impurities, whether exposed by sublimation or present from adsorption, might interfere with further sublimation — a kind of mask. Once you have a pit, the different surface area might also affect the sublimation rate.
Well, this is also just a guess, but aside from what swansont said, wouldn't water molecules along crystal grain boundaries and in crystal defects be slightly higher energy than those in regions of perfect crystallinity? Rate of sublimation would therefore be slightly higher along the crystal boundaries (which are extremely numerous unless you go to great lengths to freeze high purity water very slowly).
0 -
either this guy is incredibly thick or he's a subtle troll who's rolling around on the floor laughing right now.
0 -
Look up the hammet acidity function. In determining acidity, regardless of solvent, what matters is how strongly the acidic hydrogen is bonded to the rest of the acid.
Believe it or not, water is quite capable of behaving as a base. You will never see free [ce] H^+ [/ce] ions in water to any appreciable degree because free protons would be the strongest possible acids by definition. Instead, you have a reaction with the water, forming the hydronium ion, [ce] H3O^+ [/ce], which is the strongest acid that can exist in aqueous solution. Any acids that are stronger than hydronium ion will effectively completely react with water to give hydronium ion and an anion.
[ce] HX + H2O -> H3O^+ + X^- [/ce]
A 0.01F solution of [ce] HNO3 [/ce] and a 0.01F solution of [ce] HBr [/ce] in water will have effectively the same pH. In other solvents, this will not be the
If you want to consider how dangerous things are, you need to consider a whole slew of other characteristics. The ability to concentrate acids is important. concentrated sulfuric, for example, is a very powerful dehydrating agent and has a very strong heat of hydration (add water and it gets very very hot), which makes spills extremely dangerous. Hydrofluoric acid, since it reacts will all sorts of things, and is extremely toxic to life, has it's own dangers even though it is a weaker acid in aqueous solution than many other acids. Nitric acid is a very powerful oxidizer, so like hermann said, it will put holes in many metals or react violently with organic compounds (and release clouds of toxic gas in both cases). These same metals might be completely immune to concentrated sulfuric or hydrochloric acid.
1 -
Porous ice would be very good at adsorbing smells, not absorbing them
 1
1 -
Personally, I'd wait for spring and the ice to melt.
Apples don't grow during the winter!
But that probably isn't it!
 ~Pan
~PanYou won't get any apples during the spring either. They're a fall crop.

I would sit very still and quietly and wait for the non-zero probability event to occur where my entire body would simultaneously and concertedly tunnel to the other side of the river, allowing me to get the apples
 0
0 -
I edited some of my text while you were typing that. Sorry

I'm at home for the summer and scifinder is dead as a doornail off-campus as well.
0 -
Well, you can't argue with published procedure. I do not have access to those resources, but I'll take your word for it. Since I am in college and am heading for a career in organic synthesis, would you mind running through a general procedure for monoprotection, in PMs if you'd prefer. Do you use 1eq of the alcohol in an inert solvent with acid catalysis?
I rather like to have a realistic procedure, even if it is just a theoretical exercise. It's good practice.
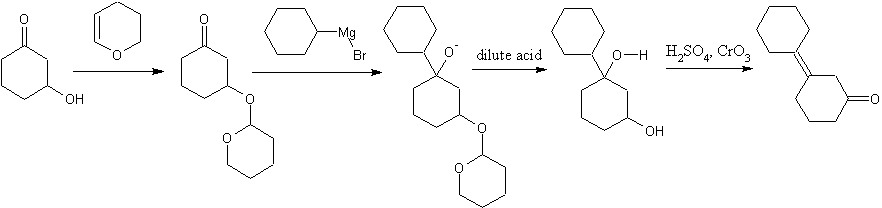
Instead of THP, how about trapping one of the ketone functionalities as an enol ether with TSMCl? Or will that be unstable to grignard reagents? a dash of fluoride in the acidic workup and any diastereomer related problems go away.
0 -
You will not get allylic bromination on cyclohexanone. Ketones are rapidly halogenated in the alpha-positions by an electrophilic reaction involving the enol. Allylic brominations are usually done with N-bromosuccinimide in nonpolar solvent, where you will generate precisely none of the enol. Who's to say what side of the enol the bromine would attack anyway. You would get a mix of 2 and 3-bromocyclohexanones if it worked.
Also, you have the hydrolysis of a secondary alkyl halide with NaOH. If you've covered the E1,E2, SN1, and SN2 mechanisms, you know that secondary alkyl halides are fair game for just about any reaction mechanism, so you will probably get some elimination as well.
I'm pretty sure that you will get borane addition across both bonds, not just the one, adding an extra dehydration step.
You can come into chat if you'd like to discuss in person. Hit the "chat" button on the top menu bar.
Are you required to start with cyclohexanol?
0 -
You won't be able to efficiently monoprotect one of the two ketones. Protection is done in an excess of alcohol with acid catalyst to ensure it goes to completion.
An ether protected 3-hydroxycyclohexanone is your best option, although using PCC to oxidize an alcohol is a waste unless acidified dichromate or another cheapo oxidizer won't work. PCC has the lovely distinction of having both pyridine, hexavalent chromium, and is typically used in dichloromethane. Acidified dichromate may have the hexavalent chromium, but has no need for pyridine or toxic organic solvent.
0 -
*cries in his soup*
How will I ever get by without that annoying noise I ocasionally hear in the chatroom!?!?!
 0
0 -
the field would be effectively equivalent in all directions, and the net field would be 0.
0 -
I actually think I got it to work regardless, and a friend and I think that the precipitate from my first attempt is Nickel (II) Hydroxide. I realize that it's not magic, but theoretically it should work, and I believe it did. Besides, I'd already seen on here in other posts that it had.
Nickel!?!?
0

Kmn04 + CH3OH
in Experiments
Posted
KMnO4 is a very powerful oxidizer and would normally oxidize an alcohol to the carboxylic acid, but formic acid is itself oxidation sensitive and readily converts to carbon dioxide and water.
If you refer to the hypergol of KMnO4 and glycerin/glycerol, no it won't work with methanol. Methanol and it's oxidation products are too volatile and would boil off before reaching ignition temperature. Generally speaking, it needs to be a polyol of some kind.