Everything posted by Tom Booth
-
Is Carnot efficiency valid?
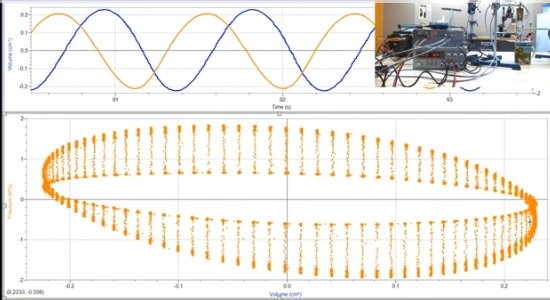
Let me ask what I believe is a pertinent question as when it comes to mathematics, I got as far as trigonometry. Differential calculus and such is a bit over my head, so perhaps there is a gas law expert or two or more here that could figure this out. You stated above: There is this PV diagrams that was generated in real time taking real measurements using an LTD Stirling engine very much like the one I used I'm my experiment. The horizontal line at 0 (left hand side of the graph) cutting through the center of the ovals represents atmospheric pressure. The narrower oval in the center area was generated with the engine running no-load. The larger oval was generated with the engine under load. These are actual readings taken in real time. The diagram indicates that both under no-load and load conditions the internal pressure of the engine falls below the outside atmospheric pressure. I have confirmed this with the researcher who did the experiment. The center 0 line cutting through the middle is atmospheric pressure and the internal pressure, especially under load, (with addition shaft work, aside from simply rotating the flywheel) drops below that line. My question is, I would think that such a drop in pressure would corelate with a corresponding drop in temperature as well. The problem with taking temperature readings is that with rapid compression and expansion the working fluid temperature fluctuates rapidly and there are apparently no probes responsive enough to record these fluctuations in real time. However, using the gas law, could the temperature at any point on this graph be mathematically calculated? My assumption is that cooling from expansion work causes a drop in temperature which subsequently results in the recorded pressure drop. Of particular interest is the increase in the pressure drop which to me implies an increase in the temperature drop with the increase in work output. Also, would the pressure falling below atmospheric pressure not also indicate that the temperature must have also fallen below the atmospheric (ambient) temperature?
-
Is Carnot efficiency valid?
You've written a lot there. As I see it, the formula under question is laughably simplistic and straightforward. A high T a low T. Supposedly the macrocosmic ∆T reduplicated and applied to the microcosmic temperature difference determines the limit of efficiency. Example: When confronted with such apparent mysticism, perhaps I can be forgiven for being a little skeptical. As far as I know, the above portrayal is not contested. Is that not an accurate representation of how the Carnot limit is arrived at ? The Macrocosmic ratio on the absolute scale reapplied to the ∆T dividing the temperature difference proportionately ? Please, if that's not how it's done I'd like to know. As far as your quote following "we look no further than here", I've already explained, that was a Joke. You fell for it way too heavily, hook line and sinker. By adopting the "Carnot theorem" POV I attempted to show the irrational consequences. How can the seemingly contradictory consequences of having all the heat flow into a 0°K "cold reservoir" at 100% Carnot efficiency be reconciled? It appears to contradict conservation of energy. 100% conversion to work AND 100% "waste heat".
-
Is Carnot efficiency valid?
I'm not "running scared of the isothermal assumption". The term is simply inadequate for describing the processes involved in a real Stirling heat engine operating at speed. A functioning heat engine is not a "bucket of ice water" or a fish which are certainly appropriate examples of isothermal systems. By "impossible", I think it should be obvious I mean; as applied to any REAL heat engine. It can of course be applied to a Carnot engine just as it can be applied to the Flintstone mobile. I'd like to remind you of the topic: "Is Carnot efficiency valid" My experiment, insulating the cold sink of a Stirling engine, that introduced the thread was not intended to prove the Tom Booth theory, It was intended to prove or demonstrate the Carnot Limit or at least one of the assumptions that follow from it. The generally accepted interpretation, apparently universally taught and assumed true of the Carnot Limit equation as that a 20% efficient engine must in the strictest legal sense of an absolute requirement, "reject" at a minimum, the other 80% "waste heat" to the "cold reservoir". The expected result of the experiment, which was to eliminate or reduce to a minimum, the possibility of such waste heat rejection, was that the waste heat would build up behind the insulation destroying the ∆T, and the engine would be unable to complete a cycle or at least soon cease operating, if able to run at all under such circumstances. The expected result was not observed. As flattered as I may be that you are interested in hearing my personal musings regarding possible alternative theories to explain the observed results, I'd like to return to the actual topic of the thread. How can the Carnot Limit actually be demonstrated or tested experimentally? My simple methodology of insulating the "sink" to prevent heat "rejection" to the "cold reservoir" apparently did not produce conclusive results. Some suggestions, I think, for improving procedure have been made, so I think the focus should really be on future followup experiments. My own theories are really off topic and irrelevant. The questions you ask were also asked about a year ago on the Stirling engine forum and my answers are there "for all to see". Is there something to prevent anyone here from following a link? Anyway your "choice" of either isothermal or adiabatic is, I think, inadequate. Like asking a Native American to choose between White or Hispanic to describe his nationality. If you are really interested in my analysis of how a Stirling engine works, does the forum support private messaging? Or perhaps in another thread. I'd really like to end the derail and get back on topic if you don't mind.
-
Is Carnot efficiency valid?
That was the unfortunate choice of words selected by my phone's over confident and overzealous and lacking in programming for thermodynamic terminogy auto-spell that I'm constantly having to fight with. Isochronic Isochronic I just wrote Isochoric those two times and it changed it each time. If I catch it and change it back it will usually leave it alone but I failed to catch it this time as I was in a hurry, sorry. As I said I'm VERY busy.
-
Is Carnot efficiency valid?
If you read my last post, I stated, among other things that isothermal processes are generally recognized as impossible, so no I do not concur, not at all. Sorry. I'm extremely busy today, but have a moment to say that much. I'll explain in more detail if I have time later this evening, as I've already stated. I do have I think a graph of the general cycle as I see it. I may get a chance to post or link to before then.
-
Is Carnot efficiency valid?
Perhaps. Certainly worth experimenting with, IMO. There is obviously some heat loss to ambient due to the piston (in an open atmosphere, rather than hermetically sealed engine) doing work on the outside air during expansion to displace the atmosphere occupying the cylinder. The "work" to push atmosphere out of the cylinder is transfered to the air outside the engine, but the heat is returned when the atmosphere reclaims that cylinder driving the piston back to it's starting position. Taking a look at the arrangement of the engine, this work/heat does not influence the cold plate heat exchanger. Not directly anyway, it is "exhausted", above the cold plate. To have the cold side of both engines adjoined, the power cylinders would need to be relocated, probably to the side, such an arrangement (with the piston off to the side) is not unusual. (Or perhaps rotation could be reversed and the bottom plates adjoined, thinking briefly on that, it might be the best way to go if there isn't some catch). Thinking about it a bit more, your idea is quite brilliant. My problem running a Stirling engine on ice has been how to isolate the ice from ambient heat. Using a second engine as "insulation" is a brilliant idea. Maybe a CUBE of 6 engines would achieve complete isolation of the "cold hole" (Tesla's term for a heat engines self-generated "sink".) It solves an additional issue I've grappled with. How to insulate the engine while keeping the power piston exposed to atmosphere. There may be some minor issue with displacer action due to gravitational pull. A Ringbom displacer arrangement should resolve that easily though. (Ringbom engines use the engines internal pressure changes to actuate the displacer.)
-
Is Carnot efficiency valid?
The theory I'm currently entertaining, after 15 years of research and observation (intermittently that is as time and resources permitted, since about 2007) is this: A Stirling engine, rotating in the same direction in either case acts as either an engine OR a heat pump depending on if it is being driven by heat or by a motor, respectively. (Or both engine and heat pump simultaneously) The "ideal" of isothermal expansion and compression is, I think, a generally recognized impossibility. (I can cite numerous sources to thet effect if need be) generally. And, Isochronic heating and cooling are also impossible, for a number of reasons, at the points of the cycle they are supposed to take place, in a Stirling engine of modern design specifically, for a number of reasons. For the past 15 years I've been researching how Stirling engines work with the original intention of building one while staying on my land in a remote location beyond power lines or utility services generally. I did eventually have an underground phone cable extended to the land but for power relied on a generator, wind or solar or "peddle power". None of these sources of electricity proved to be adequate, but I was cooking and heating with a wood stove continually so a heat engine seemed like an attractive alternative. No power producing Stirling engines, however we're available for purchase anywhere at any kind of reasonable price. And so the necessity of learning how to build one myself was both a matter of circumstance as well as a matter of basic survival. I don't have time at the moment to elaborate in detail on what I consider how a Stirling engine ACTUALLY operates, but maybe I'll have more time later this evening. To answer your immediate question though, placing two engines together with their cold sides adjacent one another is an interesting idea. However, many people have a habit of blasting a Stirling engine with more heat than it can handle or easily convert to work output. On top of that, the engines are generally given no actual shaft work to perform, so the engine becomes flooded with heat. With such an arrangement some careful load balancing would likely be a necessity. But that is also true generally, but with an "infinite" ambient sink, generally not fatal. More like $20
-
Is Carnot efficiency valid?
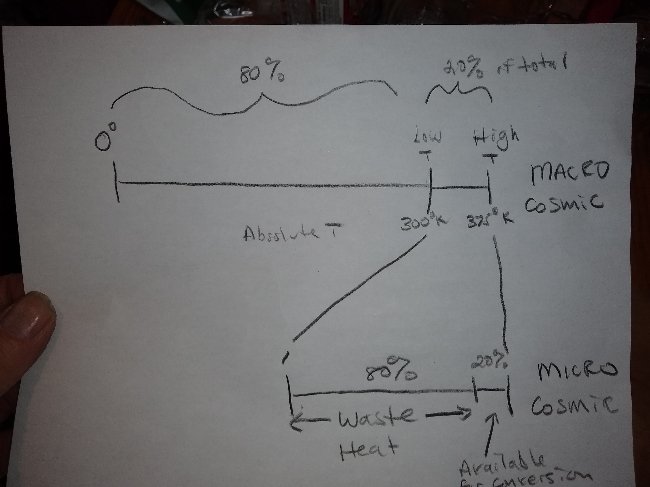
The Carnot limit equation and it's resultant "efficiency", is actually nothing but the temperature difference transmuted by mathematical trickery into a percentage of the absolute temperature scale. In other words, starting out at ambient, (300°K) we elevate the temperature to,let's say 375°K (slightly superheated steam). In the process we just elevated the temperature 20% on the absolute temperature scale. Is it a coincidence that Carnot efficiency at this ∆T is also exactly 20% ? We raised the temperature 20% on the Kelvin scale and brought the "Carnot efficiency" up exactly the same percentage. This is true at any and all temperature differences. The "Carnot limit" is IS the temperature difference. Is this a measure of efficiency? The best we can do is utilize the heat we put in and in the process bring the heat back down to the 300° ambient baseline. The only "available heat" for conversion to work is that heat used to create the temperature differential in the first place. How can a percentage of a temperature scale have anything to do with the actual efficiency of the engine. (It's power to convert "available heat" into "work"). If we convert all the heat we supplied back into work we are just back where we began, at 300°K and "Carnot efficiency" falls to zero. But we have not violated conservation of energy. We have not taken out any more than we put in. But, someone at some point in history misunderstood this basic principle and took the results of the equation to represent a percentage of OUR supplied heat. As if when we elevate the temperature 20% on the absolute scale we can only utilize 20% of the energy we just supplied. The other 80% of the heat captured, stollen, by the imagined "cold reservoir". This is insane. Some professor teaching thermodynamics made a blunder, maybe 150 years ago and this nonsense has been perpetuated ever since. Some numbskull who didn't understand that the equation only represented the actual temperature difference, the elevation of temperature on the absolute scale made a blunder. Carnot "efficiency" is, if anything, just a measure of how far down the absolute temperature scale it is before you end up back where you started before adding heat to go up the absolute temperature scale. If you went up 20% of the way (on the absolute temperature scale) you can only go back down that same 20% of the way (on the absolute temperature scale). Use some common sense.
-
Is Carnot efficiency valid?
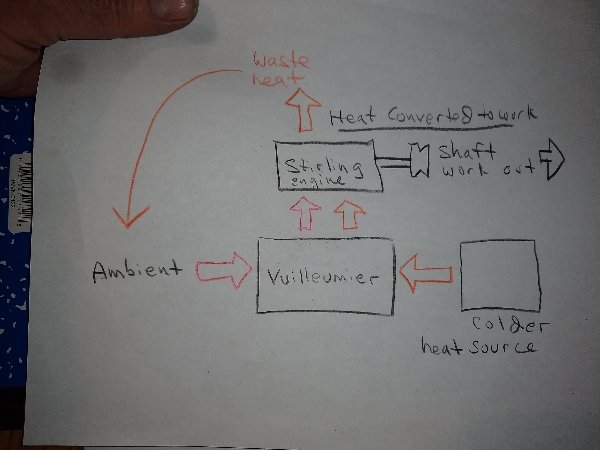
The Vuilleumier machine however produces only an elevated heat output, It does not generate very much mechanical power output, just enough to operate it's regenerative displacers, if that. Some are driven by a small motor that takes very little outside power but others drive the displacers by their own internal pressure changes and are entirely heat driven. Having no mechanical power output the Vuilleumier machine does not convert much if any heat to any other form of energy. It outputs HEAT exclusively. A Stirling engine CONVERTS heat to mechanical power output at very high efficiency. A combined Stirling/Vuilleumier machine could theoretically take in heat from both a hot and less hot source outputting the heat at a temperature higher than either, convert the high grade heat to mechanical power output. If there is "excess" "waste heat" this could be "rejected" to ambient, rather than to the colder heat source. At least I have difficulty tracing out the reason why that should be "impossible". A quick sketch to illustrate: Initially, of course, this would require expending energy for running a refrigerator/freezer to create a ∆T to operate the Vuilleumier heat pump. Vuilleumier machines ordinarily operate with very high thermal input however, like 1000°F not ambient, drawing heat from ambient as a secondary heat source, then "rejecting" the combined high grade heat back to ambient. The high grade heat output however requires continuous cooling. Such speculations however are not the issue at this juncture. The issue is the "Carnot limit", which I don't necessarily thing is "wrong" exactly, but I think perhaps it has been misinterpreted somewhere along the way, historically, making it much more restrictive than it actually is.
-
Is Carnot efficiency valid?
You don't seem to comprehend the actual aim of the experiment To completely eliminate all possibility of transfer of any heat from the engine into any sink or "cold reservoir" outside the engine. What you propose is the complete opposite of that: "...using the ocean or a lake etc as the cold reservoir would be really good." Not! I've often wondered; IF the engine is actually pulling some heat from the cold side (less hot side) of the engine, as well as the hotter side, (In a manner similar to a Vuilleumier machine (a heat driven heat pump very similar to a Stirling engine) what would happen if the temperature of the "sink" side (theoretically really just another heat SOURCE or less hot side) were gradually increased after the engine was up and running An additional heating element on the cold side as Ghideon suggested would make it possible to investigate that. The Vuilleumier heat pump draws heat from both hot and cold sides using heat as it's power source as well.
-
Is Carnot efficiency valid?
I think it would be great! Having additional control over the temperatures of both source and sink to dial the temperatures up or down would be fantastic. To begin with, seems like years ago now, (July 2020 just checked my YT uploads) I just put a piece of 1/4" foil face styrofoam over another engine, recalling an "argument" on the Stirling Engine forum from back around 2012 I think (February 2010 actually, it's still there.) about insulating the cold side of the engine. I had theorized that if expansion work had a cooling effect on the working fluid. Perhaps the working fluid was getting colder than the "sink" itself (sink = ambient outside the engine) and insulating the sink could, perhaps, increase the ∆T by blocking heat infiltration by ambient heat. That is, if the engine could increase its own temperature differential as it ran, insulation might actually help it to do that BETTER! Well, this was too radical an idea for the forum at that time. Everybody basically laughed and tried to "educate" me on how a Stirling engine absolutely MUST have a way to dump unused/excess heat or it would overheat and stop immediately. But... But... I was reading books on thermodynamics, refrigeration, gas law, gas liquefaction etc. at the time. The BOOKS said having a compressed gas expand to push a piston was an effective method of reaching cryogenic temperatures. The gas would liquefy right in the cylinder. Typically at very high compression, like 100 bar or whatever to get down to -250° or whatever to liquefy oxygen or nitrogen or some such thing, but... A Stirling engine, in principle, does the same thing. The piston compresses a gas, then the gas expands doing work to drive the engine,... Just like a compressor/expander liquefies air. How could it NOT be producing a very marked cooling effect. To me the conclusion seemed inescapable. Turn the engine over with a motor and it immediately becomes a Cryocooler/air-liquefier. Known fact. There are heat driven heat pumps as well, so why is this so inconceivable? Anyway I gave up and pretty much forgot about that debate. Ten years later I'm fooling around to see how long a Stirling LTD will run on ice or hot water and what difference insulation would make, then recalled that old idea I had pondered on and argued about 10 years earlier, and with the materials there on hand already I decided to settle the argument and put a piece of insulation over the cold side of an engine running on hot water. I had cut the insulation out previously for some other reason, don't recall what. I thought, I might as well record whatever happened and post it on the forum. I fully expected that the engine would stop almost immediately. I'd go back to the forum and admit I had been wrong. Everybody was right. I was wrong. So I watched and waited for the inevitable. The engine would overheat and stop. But it kept running. ... And running. It didn't even slow down as far as I could tell. Reviewing the recording I found that instead of slowing down, the engine actually ran a little faster. Nothing dramatic, but counting the revolutions with a stop watch, the engine was running faster AFTER the sink was insulated. It also, I found, ran longer and seemed, by the clatter it made, to have more torque and power causing an audible "knock" like the piston was being jerked inward with more force. (The knock was on the contraction stroke). If the engine had just stopped that time, that would have been the end of it and I wouldn't be here now. Sorry but I just got up. I only have time to respond to so many posts, usually in the order they appear. I was responding to a post by someone else before yours. Nothing personal, but I don't have time now. What was your vitally important question/statement again? Specifically ? I did go back and start reviewing your posts from the begining of the thread. You repeatedly insulted me and said this thread was a waste of everybody's time. I'm still working my way forward. I'm not offended BTW, my character is often questioned and I have thick skin. Skepticism is a must really, and I'm not here to have my ego stroked, obviously, I should think. Keep your pants on. Have some patience and try not to act like a sniveling spoiled punk that wants to be the center of attention of throws a fit like some 2 year old and BTW some of your posts make no sense. Be glad I DON'T respond in most cases. As my mother used to say, if you don't have anything nice to say, don't say anything at all. If you think this thread is a waste of time, I'm sure there must be other threads in this forum somewhere.
-
Is Carnot efficiency valid?
"Cold reservoir" or heat "reservoir" generally is a product of Sadi Carnot's imagination. You, or someone in here said I'm obsessed with "caloric theory" which has long ago been abandoned but the language of thermodynamics harkens all the way back to it and the language influences thinking on the subject of heat still to this day. What is in contact with "the plate opposite the hot plate" in the last experiment was silica Aerogel and beyond that the surrounding ambient air. You said: Implying the "reservoir" should be something other than the ambient (which is "colder" than the ambient). Talking abstractions like "reservoir" is fine when discussing theory but this is actual, not some thought experiment, this has to be implemented in the real world in real life.
-
Is Carnot efficiency valid?
I guess I automatically thought of "0°C" as the freezing temperature of water, so if not ice, than ice-water. Sorry for the misunderstanding. You said "the cold reservoir" should start out colder than ambient, perhaps 0°C What "cold reservoir"?
-
Is Carnot efficiency valid?
This was your earlier suggestion. I read: "no ice". Are you suggesting conducting the experiment in an ice box ?
-
Is Carnot efficiency valid?
Not ignoring you Studiot. Just responding in general. The purpose behind the experiment, originally, is to see what happens to the engine with the "sink" blocked. The "natural" existing sink, (if we are going to apply steam/heat as the heat source) is the ambient atmosphere. Introducing ice or ice water creates a mixed sink. Partly ice or cold water and partly ambient, and it would be difficult to distinguish what's what, I would think, and the heat melting the ice or warming the water can then be originating from multiple sources: the ambient surroundings, the heat from the steamer or the engine itself. But, IMO additional data does no harm. Experiments can be run over and over in various ways. Sometimes unexpected results emerge. My only problem is finding the time, so I try to make experiments as simple and quick as possible and with meaningful results. I'm not just experimenting for the sake of experimenting to waste time. My question I want answered is where is the "waste heat" that the Carnot (so-called) equation suggests should be there. So how does melting ice, or warming cold water help answer that question?
-
Is Carnot efficiency valid?
Melting the ice? I thought you said NOT to use ice. Perhaps a miss-statement? Ice can be below 0°C possibly different temperatures at different parts. (Extremity, center). I had assumed the idea of only using near 0° ice water was to avoid all that potential ambiguity. The ice could take in unknown quantities of latent heat before begining to melt. But you did say "ice water" above. Rather than speculate, I'll just allow you to clarify. If the goal is to only change one parameter at a time. Why change the given (ambient) temperature of the cold side ? To my mind, as I said before, this just introduces an additional needless variable to no purpose rendering any results ambiguous. Aside from the obvious clarification needed. Why? Seems like redundant data unrelated to the issue under investigation. Or if you believe it is related, how so?
-
Is Carnot efficiency valid?
When I have more time I'll go back and read through all this mess, right now I'm trying to run wires, install breakers, drill holes etc. so my wife can use the kitchen. There's quite a few questions I've asked in here that haven't been answered, and other posts beside yours I need to go back and address as well. aside from the experiments I'm trying to squeeze in.
-
Is Carnot efficiency valid?
OK. I don't personally see what difference it makes what the starting cold side temperature is so why not? Put some ice water on top of an inoperative engine (heated by the facial sauna or some other heat source ???) and measure the temperature change, plot a graph. Sounds interesting. Then do it a second time letting the engine run. What about the Arduino. Do you agree the inoperative engine should have the displacer actuated by a servo motor? Right. The routine experiment every physics student knows is very very super secret. Every student has to sign an NDA and promise to destroy all notes and materials before leaving class.
-
Is Carnot efficiency valid?
Certainly if such experiments are carried out continually on Colleges all over the world on a routine basis, there should be some well worn procedural outline to follow. Materials required, setup, etc. There should be textbook procedures to follow. Every student gets a little engine to test and some temperature probes. To say "It’s not noteworthy, so nobody is going to publish these results." Is IMO absurd. No disrespect, but if that is the case the procedure should be well known and available. Published somewhere.
-
Is Carnot efficiency valid?
Not sure what the relevance of your work history is to the little LTD Stirling operating on a the steam from a facial sauna. The formula in question is the Carnot efficiency formula. It is based on the temperatures of the steam (something under 100°C and ambient sink (about 70° F) Do you contest that the Carnot efficiency would be about 20% or less ? That the heat rejection would be 80% or more ?
-
Is Carnot efficiency valid?
At zero efficiency then, 100% of the heat should go straight through to the sink ? The top of the engine should get as hot as the bottom heated side. Correct ? But IMO the working fluid IS doing work driving the piston, creating friction, agitating the air, making noise, doing "work". The discussion is about Carnot efficiency. Occasionally I forget to insert "Carnot" before the word "efficiency" but in most cases in this context I'm talking about Carnot efficiency. What are you talking about? This thread is moving very fast and I'm trying to get off here for a while to get some things done today. Sometimes there is cross posting and I miss something but I'm not "stopped talking" to you again. Assuming you are addressing me. The above post? Are you arguing that keeping some account of energy input is impossible or something? My electric company sends me a bill every month. It's not rocket science.
-
Is Carnot efficiency valid?
Small flat ceramic heating elements. Could slip that right inside the engine above the bottom plate to heat the working fluid directly. Can run on a 5v USB charger or less. Maybe a few AA batteries. Possibly. Not entirely sure. I haven't had time to look over all the specs. There are all different kinds with different input voltages and outputs. "Wrong" what?
-
Is Carnot efficiency valid?
Just as I stated previously. You seem to just want to argue and engage in hair splitting. My experiment was simple and straightforward. "Carnot efficiency" predicts, according to multiple online sources, that an engine operating at the temperatures used should at best, being very very generous "reject" 80% of the heat to the cold side of the engine. Much more actually because the actual ∆T is considerably less than my estimate. calculated based on 212°F and 75°F. The steam reaching the engine was probably what? So actually 90% ? 95% waste heat ? What would you estimate we should find at the sink? My IR camera barely read above ambient after 15 minutes at the top of the Aerogel blanket. What do you conclude from that? Why doesn't the engine overheat in your opinion, when the sink is insulated ? I know, Aerogel is no different than air, or it conducts heat or absorbs it or something. Fair enough. I'll drill some holes and insert temperature probes as you advised. Sounds like a great idea to me. What is the predicted outcome? What about something like one of these: ?
-
Is Carnot efficiency valid?
Sorry, I didn't realize that was a screenshot of something you considered important. I don't. I can take a look if you want, but what's supposed to be significant about it? Not my diagram. But at a glance, it looks like an energy flow diagram of the drinking bird. Give it a push. User input (starter) Environmental input: heat on the bottom end. Irretrievable loses: friction etc. Output: mechanical energy and cold air. The birds cold head does cool the air some I suppose, and there is some mechanical activity going on. Recycled heat? Not sure about that. Why bother? The environmental heat isn't going anywhere. Though lets think about that. Some friction results in heat which going into the environment might find its way back to the birds abdomen. Maybe performance could be improved by attaching a flexible heat pipe between the pivot bearing and the abdomen. Would that actually raise the input temperature any? Not likely IMO. Heat locked away in the cold air/evaporating moisture? Any way to get the water to condense and use the heat of condensation? I wonder. Anyway the bird operates without all that additional potential heat recovery. Don't see where such a chart should alarm anyone or be considered particularly extraordinary or controversial. Am I missing something? Anyway, I'll likely be busy the rest of the day. I've promised my wife I would install some additional power outlets in the kitchen. She's tired of running appliances off extension chords. Also the living room needs sheetrocking and additional outlets, not to mention the usual daily chores I've been neglecting.
-
Is Carnot efficiency valid?
What am I doing here? I would say it is something like "peer review" if I were a scientist. I did an experiment and thought the results were rather curious and worth sharing to see what others think about it. It was mentioned by a moderator that I have to make all the details of the experiment known so that others can replicate it. That would be interesting and much appreciated. Getting others points of view on experimental procedures and ways to improve the experiment are valuable, potentially. I would continue to share whatever progress is made and post additional experiments, if there is an interest. I'm not trying to "prove" or "disprove" one POV or the other. Experiments don't have, or are not supposed to have a predetermined outcome. Academia (the various online educational resources) all use the Carnot equation as I have here. If 500,000 Joules are provided to an engine at the given temperatures 400,000 will be "rejected" into the sink. Can this actually be verified experimentally or is this teaching just being perpetuated generation after generation without anyone ever actually taking the measurements? You mean like the drinking bird? That is an actual thing you know. Calculating FUEL efficiency is pretty straightforward. Miles per gallon. Carnot so-called "efficiency" is something all together different. Unfalsifiable yet inescapable. Power plant, turbines whatever. Carnot efficiency is calculated from a temperature difference. Suppose a turbine puts out cold air below ambient. How would the Carnot efficiency of the turbine be determined, I'm curious to know. Working as a mechanic I once got a job that started as a temporary position replacing an employee who had an accident. The accident was he was using an air impact wrench and broke his finger off because it had been cryogenically frozen by the exhaust from the air tool he was using. So, a turbine exhaust using compressed air can be well below ambient. Is this correct? What temperatures then would be used to determine Carnot efficiency?

.jpg.172481fd9481a756d80f44edc85ad951.jpg)