Everything posted by Tom Booth
-
OT from The "Ice Bomb" thermal engine
I think I can legitimately state with absolute honesty that About 10 or 12 years ago I 100% accepted that a heat engine operated by shunting heat from the hot side of the engine to the cold side. It made perfect sense to me. I was on the Stirling engine forums asking questions, reading, and just wanted to know how they worked so maybe I could build one to run off my wood stove. I studied, read, watched hundreds of videos and had no reason to doubt the accepted, consensus wisdom on the forums. If you heat up air it expands and pushes the piston. If you then cool down the same air it contract and the piston can be pushed back by the momentum stored in the flywheel. Nobody at that time had ever imagined that a heat engine could run without a flywheel. But in time I saw things about heat engines that made no sense to me. That made me scratch my head trying to figure out how that could be possible. One guy accidently discover his engine could run without a flywheel. Some engines just seemed to run too fast. It took like five minutes to get the engine hot enough to run, and it would run five more after the heat was removed, but ran at 3000 rpm or something. Heat just doesn't conduct through a steal plate that fast could it? Well, so I started reading up on thermodynamics, but had to settle for whatever old textbooks I could find on the subject in libraries or online and such, so, maybe some stuff was obsolete. What struck me was when I read about how heat can be converted into work in a heat engine, and that as a result the heat actually "disappears". It's no longer inside the engine but went out as "work" so in actuality, did not need to be removed. How amazing is that? Well that really explained a lot of my observations that were puzzling me. Heat when converted to work "disappears" instantaneously. Or some old texts related. So THAT'S why some engines can run so fast. So THAT'S why the flywheel isn't needed to push the piston back down the cylinder. In some engines, though, the piston returned with more force than what the heat added could force it out. In other words, it seemed that air was expanding so far, then coming back on it's own with so much force it crashed into the HOT end on its own. That looked impossible. So in trying to figure out that one I thought well, maybe so much heat is being removed as "work" that the air in the engine is getting colder than it normally would. Let's see. Heat pushes the piston out as far as it will go, as far as the gas will expand, but the piston is heavy and has momentum, so the momentum carries the piston further. But the air has already expanded as much as it wants, so now the piston's momentum is stretching, or pulling, "expanding" the air more. There are a lot of cooling processes going on simultaneously. Heat converted to work, mechanical expansion, there is an air passage the air passes through, an orifice. Joule Thompson throttling. Why not anyway? Heat drives the piston out to the full extent possible. Energy is stored as momentum. The stored energy then does more work. Then as the air cools and comes back, the piston, (that engine had magnet attached to the piston) passes the coil generating electricity, which is more work being done as the air is already contracting, and, well, the engine is moving so fast, no time for heat to conduct out through the sink, so with all that cooling maybe the sink is not needed. Maybe the air in the engine is actually getting COLDER than the sink! Well with that inspiration, I returned to the Stirling engine forum, and basically they all just laughed and tried to explain how a Stirling engine works, and NO absolutely not, putting insulation on the cold side of the engine won't help it to run better. The heat has to go out to the sink to cool the air (inside the engine). Otherwise the engine won't run. A Stirling engine can't run at all with the heat sink insulated in some attempt to keep the atmospheric ambient heat outside from going backwards into the engine. But... But... I was pretty sure I was right. If the sink were insulated so heat couldn't enter from the sink/ambient/cold side then it might be able to refrigerate itself more and run better, gradually getting colder and colder, instead of the heat building up like water behind a dam, it would be converted into heat more efficiently. Well, I didn't have an engine to carry out my experiment. But I prodded the model Stirling engine builders who HAD engines to try it on to try it. None ever did. Not worth bothering, we all know it can't work ha ha. Well, I put the idea on the shelf for another ten years, until an Uncle died and left me some money, so I could just go out and purchase a half dozen engines to experiment with. Finally I did my experiment, after wondering about it all those years. I thoroughly Insulated the sink side or the engine so no heat could pass through, set it on a hot cup of water and gave it a whirl, expecting everybody was right and it would just come to a stop because the heat had nowhere to go. No way for the engine to cool back down. But it ran. And it kept running. I timed how fast and how long. I ran "controls" with identical engines. The insulated engines ran longer at higher RPM and ran more vigorously, like it was on high octane gas or something. I'm not being deceitful or trying to pull anyone's leg. I video recorded the experiments. Then there were more and more experiments with strange unexpected results. I tried running an engine backwards on ice, and while the engine was laboring hard, because the piston I made was too tight, well the ice that had started to melt re-froze while the engine was running, and the engine got frozen on the cup of ice and I had a hard time getting it loose. When I broke it loose and got it running again, it got frozen, or refrozen. A few oddball results by one person don't prove anything, but there is a pattern. It appears that heat in these engines is not flowing through to the sink as everybody has assumed that it must for the past 100 years. I never heard of Tesla at the begining of all this but at some point I came across his article in Century magazine 1900, where he explained the results I was seeing in his refutation of Carnot I guess you could call it. But I had come to my own conclusions before I came across what seemed like confirmation from a well know person of science. Anyway, if nobody here is interested, no choice really but for me to move on I suppose and quit wasting time. That's what my girlfriend keeps telling me. "Why do you bother? Why waste your time?" I feel I'm trying to just selflessly share some hard won knowledge and information. Not charging anybody anything, all on my own money and my own time. OK so a few times I tried starting some crowdfunding to raise money so I could do experiments and build engines, but no luck there either. Seems like this "2nd Law" has everybody completely brainwashed, so they can't even look at what's right there in front of them. Won't dare look at all. Won't even think about it. Believe what you want. Nothing new. As someone pointed out, I've been at this for years. I just think it's my duty of sorts to put it out there and share with whomever might listen, or whomever might come along in the future. My latest project is a computer controlled engine to try out some things that aren't possible when the displacer is attached to a crankshaft. This is just my first attempt at programing the Arduino to work a servo to control the displacer motion.
-
OT from The "Ice Bomb" thermal engine
Sorry, I stated that the wrong way around, but maybe someone gets what I mean. If 70°F is taken to be "all the heat" 20°F is the sink. In kelvin that's a 27.778 difference. So if we divide all the heat by 100 so that 294.261K = all the heat that's 2.94261 So taking the difference and divide by 2.94261 we get the same exact value as the "efficiency" to like the tenth+ decimal place. So called maximum heat engine "efficiency" is just the temperature difference on the Kelvin scale written as a percentage? From my pov, it seems like nobody else is "getting it". I understand the consensus, (mostly) pov. I just can't say that it actually makes sense to me. Especially like, when I test something to try and find out which pov is correct and witness the results myself and I'm told my results are simply "impossible" so, nobody knows what but I did SOMETHING wrong. It's like I get off an airplane in a country where people still believe flight in a plane is impossible, and nobody will believe I just got off a plane. I can show you my plane, it's just behind those trees, we can take it for a ride, see for yourself. I have pictures, see. The pictures are torn up. Nobody will look. I've been on at least three science forums now where I was told my VIDEO shows a "perpetual motion machine" and the thread is locked because it's against forum rules or something. Seriously? It's just a heat engine folks. A TOY model engine at that. I'll send everybody one for free, do the experiment yourself. Thread locked. Wow.
-
OT from The "Ice Bomb" thermal engine
My reason for appearing here, was, a little over a week ago, I had posted a question on the Stirling Engine Forum I frequent: There was no response. So I thought perhaps the knowledgeable folks on this or another Science forum might have some input. I don't think I've received an unequivocal answer. Perhaps the equation can't be used because this engine doesn't behave in the expected way, and something about steam tables and not having a glacier or something along those lines. Would anyone else like to offer an opinion? Maybe just an ordinary heat engine? So treat it as such? T hot = ambient T cold = freezer so W should work out to be? Plug in some realistic numbers I guess. Say ambient 70°F and freezer, I guess we could make 20°F Sound reasonable? What's W? Work is it, right? Convert the numbers to the Kelvin scale. Am I even using the right formula for efficiency? 266.483 ice box 294.261 ambient Efficiency = 1-Tc/Th is it? 1 - 0.9056008101651 Is 0.0943991898349 So, the decimal moves 2 places and that gives roughly 9.4% efficiency That's absolute maximum of course right? Would that be the whole ice engine setup? If say 200 or whatever Joules of heat are taken in from ambient heat per hour then 18.8, with loses, say 10 joules of work could be extracted? Well, with friction, mechanical loses etc. 5? Converts to 3 or four foot pounds of torque I guess? (Or so an online converter spits out). Anyway, trying to be conservative, it looks like there is at least some potential for power output. Now, does that include the entire apparatus freezer and all, or does the power to run the freezer have to be deducted? I'm guessing the answer will be that the power to run the freezer has to be deducted, so there is really no point and it's all a hopeless waste of time. Right? Or is it like someone said and the whole thing is not amenable to conventional mathematical analysis? I don't know really. I'm sure I've got the math all backwards even if it were applicable. My main question was T hot is usually equated with heat input. But in the freezer, power is produced by taking heat away. So, no matter, just flip it around? I'm really not intentionally trying to make anything difficult or confusing. To be honest, the whole efficiency formula seems simplistic and meaningless. It's just the temperature difference. Literally, just the temperature difference. 294.261 - 266.483 = 27.778 27.778K is exactly 9.43991898349% greater than 266.483 on the Kelvin scale So "efficiency" it's just another way of writing the temperature difference. How does that make sense? Anyway, why should empirical evidence, or actual experiments be taboo on a science forum? Actual video and measurements, temperature readings, things of that nature. I'm much more comfortable with something I can actually run and take measurements on, but whenever I do that, the posts are sent to trash or the tread is locked. Does Science not do actual experiments anymore?
-
The "Ice Bomb" thermal engine
I don't think I'm hung up on anything. I think maybe you might be though. I just said that the engine does not convert "ambient heat" into work. It converts "chemical potential energy in liquid water" into work. I don't think it can be factually stated that the distinction makes no difference. To say that it converts "ambient heat" into work implies some continuity or causative connection between the ambient heat and the work, but I question if that is actually a fact. The energy represented by chemical potential energy could have come into existence by any number of possible means. A hot plate-(electricity), direct sunlight, agitation of the water with a paddle, a coal fire, the water could have cooled down from a higher temperature as it shot up from a hot springs-(geothermal), etc. etc. Once heat is bound up as chemical potential energy, there is no longer any "flow", no causal force passing through like a river. The "ambient heat" that may have been taken in is not the same heat, if any, that might, (or might not) be released when the ice expands and lifts a weight in the process. Ice as it freezes and expands exerts a force of some 100,000 pounds per square inch. apparently. Crystalizing ice could power a piston, six inches in diameter, to lift a weight of some two million pounds or so, if that is really the case. I'm not convinced it can be assumed that there is necessarily an equivalence there, between the ambient heat that went in and the work that could be taken out.
-
The "Ice Bomb" thermal engine
Agreed. So when we have "chemical potential energy in liquid water" sitting there. Is that a flow? Where do you see any heat? No flow, no heat. Is that not correct?
-
The "Ice Bomb" thermal engine
Thanks for taking the time to explain your point of view, which I don't necessarily totally disagree with. But in the economy of energy, we have the conservation of energy: "Energy can never be created or destroyed it can only change form." Putting that in economic tems, if I start out with US dollars and convert that to euros. I no longer have any dollars. The dollars are gone, and now I'm now carrying euros. If I then convert the Euros to Yen, well those original dollars are nowhere to be found, so if I then convert some of those Yen into British pounds and the rest back into dollars, it wouldn't really be accurate to say that I converted my dollars into British pounds would it? Really, have I even converted "some" of my dollars into pounds? No, I converted my Yen into pounds. I just think it is important when conceptualizing these things to keep in mind that HEAT is not a physical thing that "flows into" and then "flows out of" any kind of heat engine, and that energy itself is basically just an accounting. It creates a picture in the mind where what goes in, must all be coming out the other side, but that is not the case. What went in, the ambient heat is already long gone right from the start.
-
The "Ice Bomb" thermal engine
Hopefully the relevance is obvious to others. It's just an example of the conversion of heat to work in a thermally controlled, heat restricted environment. When a substance does "work" and cannot take in additional heat, from the surroundings, the work is accomplished at the expense of internal energy, which accelerates the change of state In this case, causing the water-ice to freeze more rapidly. (Perhaps almost instantaneously, like a bottle of drinking water super-cooled in the freezer can suddenly freeze when agitated), the same way a gas doing work in a COLD insulated turbine suddenly liquefies. I won't get into how all this relates to my SE experiments. But anyone here familiar with that subject should be able to put 2 & 2 together. An SE engine works by thermal compression alternating with mechanical expansion. Similar to the compression-expansion that takes place in a bootstrap turbo-expander. Isolated from the ambient environment. Thoroughly Insulated. Particularly on the cold expansion side. What happens?
-
The "Ice Bomb" thermal engine
Well here are a few additional references I'll be so irresponsible as to post about a non-existant process that the so-called cowboy operations can peruse for their entertainment. https://www.laturbine.com/wp-content/uploads/2020/12/Fundamentals-TBX-GPLNG.pdf L.A. Turbine - 28557 Industry Drive, Valencia, CA 91355 - manufactures and services turbo-expanders. http://rddynamics.com/products/turboexpand.html R&D Dynamics designs and manufactures turbo-expanders for specific applications. https://machinery-inanutshell.blogspot.com/2014/01/Turboexpander-Performance-1.html https://www.sciencedirect.com/topics/engineering/expander-process To conclude for now, here is a very in depth thesis in (PDF 175 pages): "Design and Construction of Turboexpander based Nitrogen Liquefier" It includes the entire history and historical development of all the various gas liquefaction processes with particular emphasis on turboexpanders for liquefying nitrogen. http://ethesis.nitrkl.ac.in/6598/1/DESIGN_AND_CONSTRUCTION_OF_NITROGEN_LIQUEFIER.pdf So much for "never designed for the purpose". the number of references available could fill a library. A few highlights: Turboexpanders have been used for the liquefaction of various gasses, (though I don't know your age), likely before you were born. and I don't know what your supposed experience in the oil fields in Nigeria or West Africa may have been but it is a small fraction of the many applications for liquefying gases with turboexpanders. And I'm not even talking about Petroleum. I'm talking about gases like air, hydrogen, helium, nitrogen, oxygen etc.
-
The "Ice Bomb" thermal engine
I don't know where you're coming from but Turbo-expanders are WIDELY used for gas liquefaction. It has practically replaced every other method there is in every industry involved in gas liquefaction all around the world. First you try to deny it exists, apparently because it wasn't on Wikipedia from which you were apparently cp'ing, now you are trying to demonize a standard industrial process, used all around the world. What's your game dude?
-
The "Ice Bomb" thermal engine
What? It's friggin' common knowledge except maybe for (some) in the industry who try to guard it like a trade secret. Which to some degree it (sort of) is. But do you read: "standard in the natural gas industry for liquefaction." not as you suggest "malpractises". At any rate a long way from "Never".
-
The "Ice Bomb" thermal engine
Who I was responding to is right there in the quotation box. How about you stop devolving the conversation into petty attempts at character assassination. If you are unfamiliar with such a use for turbo-expanders, I can provide references, if not banned from the forum before given a chance, which is usual for this juncture. We are talking about turbo-expanders used for gas liquefaction are we not? http://gasprocessingnews.com/features/202006/fundamentals-of-turboexpander-design-and-operation.aspx
-
The "Ice Bomb" thermal engine
What you are referring to is my response to exchemist, Right 🙄
-
The "Ice Bomb" thermal engine
You're referring to a different use case. That's like saying the evaporator in a refrigerator is undesirable because it doesn't produce heat or pressure. A turbo-expander when used to liquefy gases is not meant to produce work, it's meant to liquefy gases. That it succeeds in liquefying gas does not "reduce it's performance" because it does less work. Doing work is not it's purpose. Most turbo-expanders used for liquefaction of gas are bootstrap systems where the work output is used only to reduce the load on the compressor and the two are coupled together on the same shaft. There is no external work output. That's not it's purpose. How can the liquefaction of gas, in a gas liquefier be "undesirable" when that is it's purpose? Too bad that the liquefaction of gas in a gas liquefier is "sometimes unavoidable". Boy how those gas liquefier operation guys wish that they could avoid having those darn turbo-expanders goin' and liquefyin' gas all the time. How they just wish they could find some way of avoidin' that, gosh darn it. I said liquefaction. Not chilling and depressurizing. This is, of course true. The expenditure of energy, the work performed by the gas, to turn the turbine, results in phase change. You have it backwards, or are referencing a different use case. For many difficult to liquefy gases, it was found that pressurization and cooling was not enough. A "quick and dirty" method to liquefy such gases was to first cool and compress the gas as much as possible, then in the final stage, to release the compressed gas through a turbine attached to a load. The turbine is kept thermally isolated from the ambient environment so that the gas, as it expands through the turbine performing work is unable to absorb any heat. Under these unusual conditions, the gas draws on INTERNAL energy in powering the turbine. The sudden loss of energy, with no way to get it back by absorbing heat from the environment results in the instantaneous condensation of the gas into a liquid within the turbine. The process you reference is unrelated. Turbo-expanders are used in many different ways. Hmmm. Since when is saying that it is "worthwhile to consider" something "pretending expertise"?
-
The "Ice Bomb" thermal engine
It goes back to my statement that the "ice bomb engine" does NOT use ambient heat to do work at all. You said: "yes it does". My point is, no, it doesn't. Saying the ice bomb engine uses heat to expand is like saying that my car runs on sunlight. I'm saying: no, it runs on gasoline. Ultimately, it can be conjectured that my car does indeed run on sunshine, and in a roundabout way this is correct, but the reality is, my car does not run on sunshine it runs on gasoline. You can trace the potential energy all the way back to the big bang if you want, but the fact remains, practically speaking, sunlight is not the immediate primary cause that enables my car to run, the sun shining or not shining has no immediate bearing or direct influence on the operation of my car, which continues running just fine long after sunset. The "potential energy" stored in liquid H2O is no longer HEAT any more than gasoline is actual sunshine. You then go on: What? If I carry a bowling ball up a hill, it "stores" potential energy, metaphorically speaking. The potential energy cannot "flow out" of my bowling ball, or anything else. Potential energy is not a substance that can be carried around like water in a bucket and poured out.
-
Martian Hydroelectric Concept
Snow in the mountains imperceptibly melting, added together results in a raging river downstream. I can imagine something similar taking place in the pipe, where there is little action to be seen near the extreme interfaces but a considerable surge in between.
-
Martian Hydroelectric Concept
I wouldn't go so far as to day "nobody". I may not be 100+1% convinced, but neither are you, right? Or why ask if others here think it's viable or not. I was all geared up to get ready to help build a prototype. I mean, as much as I might play up my "ice bomb" engine from a purely theoretical standpoint, even I have my doubts it would actually work in practice. I'm much more an experimentalist than a theorist. I'm skeptical that I've actually been posting messages on the internet all these years. I might just wake up someday and find out it was all a crazy dream and have to go back to using the telephone. The internet is fantastic, but still seems a bit unreal to me. If I build something and see it actually work, or do an experiment and see a positive outcome, I still want some independent confirmation, peer review, replication, and probably still wouldn't fully "buy into it" The theoretical foundation for why it worked could still be complete hogwash, which is why I don't "believe" established science. It isn't that I don't believe established science. I just live in a perpetual state of suspended judgement, silently watching the world go round.
-
Martian Hydroelectric Concept
I'm wondering, why not build a small model, a loop of pipe, a heat lamp in a cold room, some flow meters in the pipeline, a stepper motor to keep it revolving. Like a planetary mobile, to provide a sense of realism. A relatively inexpensive setup I think. Hardly complicated at all. If some actual flow potential is indicated, maybe even spring for some miniature turbines. Someone could probably even 3D print the things on the cheap. Maybe get some actual power output measurements going. And, might this not actually work, (if it works at all that is), on a mid-scale, say for instance circling a mountain or building somewhere near the poles. In Alaska perhaps, where the sun never sets.?
-
The "Ice Bomb" thermal engine
Ok, well, I'm not surprised, but I would think after all these years in business, such false advertising should have caught up with them. But the fact they are still in business making the same claims, again, makes me wonder. _____________ Returning to the freezer box. I imagine, as the liquid water molecules "release" the "latent heat" it is not dumped into the freezer, rather it is used by the liquid H2O in the construction of it's crystal lattice. Internal molecular bonds or whatever, to form the ice. I question exactly how much "waste heat" would actually be released. In this respect it is, I think, worthwhile to consider the actual industrial process of gas liquefaction through an expansion turbine. The phase change draws on internal energy converted to "work" not heat transfer to the environment. And back in the freezer, the expanding ice doing additional "work" in the process of formation, probably requires additional heat which it draws from its surroundings. So actually the ice box probably gets a little colder as the ice forms and simultaneously performs "work" lifting a weight. ______________ Unfortunately it seems, if I were to construct this ice bomb engine and run it, demonstrating the factuality of these hypotheses, what would be the result if I were to post my data, complete with video, visual demonstrations?
-
The "Ice Bomb" thermal engine
"Chemical potential energy" is not heat. The original ambient heat supplied has already been converted to something else entirely. By lifting a weight in the freezer, the "chemical potential energy" is, at least in part, converted directly into mechanical energy, not heat. So in theory, less heat needs to be removed than what was originally supplied. It is removed to supply power to the load, the spring, or whatever our ratchet device is winding, converting the chemical potential energy into useable form and skipping heat release into the ice box altogether. Perfect energy conversion is probably unobtainable. Some "waste heat" will likely be generated, necessitating removal, but it will be less heat, not more than what was previously added. Theoretically anyway. Can we assume an extremely low efficiency for this ice formation? That is, is a whole lot of heat being transfered? Let's say the water warmed just enough for it to thaw is at approximately 32°F. To re-freez it needs to be cooled down to, approximately.... 32°F As it crystalizes it performs work but remains at 32° F. How much "HEAT" transfer is actually necessary to bring water from a 32°F liquid state to a 32° solid state? There are other means and methods of transferring energy other than heat transfer, which is the whole point that Tesla was trying to make. Heat is not a physical quantity that is added and removed, it is one form of energy that can be converted to other forms. In thermodynamics "work" and "heat" are equivalent. I hesitate to make the claim that ALL these compressed air heat recovery industrialists are lying through their teeth.
-
The "Ice Bomb" thermal engine
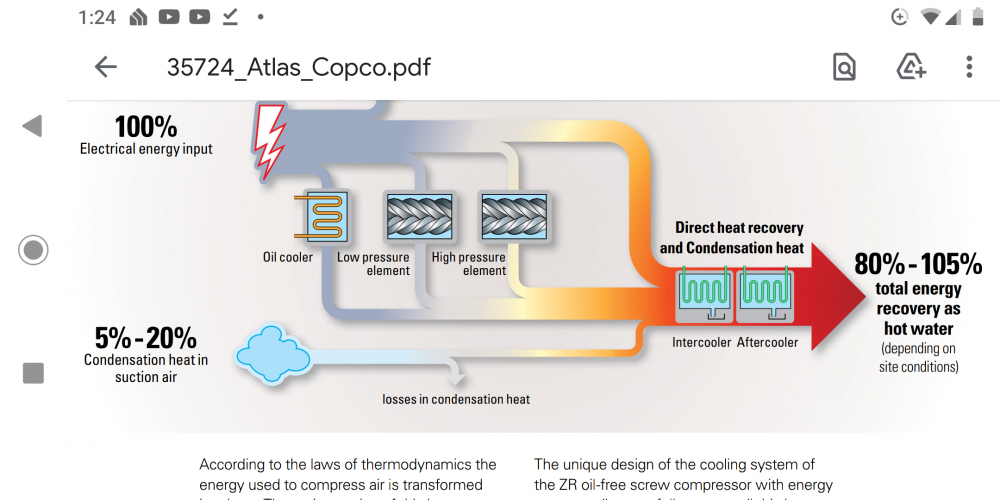
This is the confusing part, in that the ice engine does not convert ANY ambient heat into work. Does it? Heat has to be REMOVED. As stated before, hydrogen bonding, or whatever intermolecular forces do the lifting. Molecular Internal energy, not "heat". You can't have a percentage of a negative number. Remove x joules and convert 5% of this "heat input"? It's not heat input, is the point. The "work" output is not a result of any heat input. The heat REMOVAL allows an internal force to dominate, like bringing magnets a little closer together so that SNAP! A much GREATER attractive force than the tiny little nudge that brought them close enough together for the attractive force to dominate over the frictional force (in the case of magnets lying on a table) keeping them apart. That molecular attractive force that does the actual work of lifting when the ice expands is enormous in comparison with the minute amount of heat transfer taking place. Getting the magnets APPART again if where we, theoretically are at a disadvantage, in this analogy. An ENORMOUS addition of heat is required to break the bond. But we supply that heat easily, right from the surrounding atmosphere. Now the freezer/heat pump in theory, let's say, will need to remove an amount of heat equal to that which was added outside the freezer. But that heat is "pumped" directly to our heat engine and converted, with the result that our compressor need not work as hard as would otherwise be necessary, (completely ignoring the conversion of heat into work going on inside the freezer, this work admittedly, perhaps only slightly reducing the amount of heat that the compressor must remove.) In theory, the mechanical output of the heat engine can serve as our compressor to run the refrigeration system. As Tesla, indeed pointed out in his 1900 article, Air compressors can operate at an efficiency up to 96% when "waste heat" is utilized as advertised by the industry. https://us.kaeser.com/products-and-solutions/rotary-screw-compressors/heat-recovery/ That is why I think the air-cycle refrigeration system is so attractive with this kind of application. Some companies, remarkably, have boasted of air compressor energy recovery in excess of 100% under certain conditions. https://www.ien.eu/uploads/tx_etim/35724_Atlas_Copco.pdf
-
The "Ice Bomb" thermal engine
It can be confusing, but I'm trying to sort it out myself, not intentionally trying to confuse anyone. Some applicable hard mathematics or PV / entropy charts to clear things up, is what I was hoping for.
-
Martian Hydroelectric Concept
Pipe rupture due to water expansion pressure rather than displacement? In a completely rigid pipe, the water sealed between ice dams,... Lots of potential expansion and contraction issues, maybe? If the pipe is elastic to avoid rupture, that reduces horizontal displacement leaving a long pipe that just kind of throbs around, possibly rupturing and ripping itself from its mores like a loose firehouse. But that's just one of those practical challenges. I had a picture flash in my mind of a Nitinol engine. The kind where a loop of Nitinol wire revolves around some wheels. In this case, the Nitinol pipe circling the planet. Eliminate any possibility of rupture, and enhance the displacement effect, by "training" the metal to... Not sure what, but the super-elasticity might be of some advantage.
-
Martian Hydroelectric Concept
Why for only an hour a day? Space several turbines at different locations along the pipeline and generate power all day and night somewhere on the planet.
-
The "Ice Bomb" thermal engine
The compressor could be driven by anything. How it's driven is irrelevant. How much it cost is irrelevant. The compressor could be powered by a driveshaft from a paddle wheel in a nearby river. The compressor could operate perfectly, contributing no heat whatsoever. The heat from the evaporator from inside the refrigerator is still concentrated in the condenser on the outside to be dissipated. The compressor gets hot, because it's packing all that refrigerant into the condenser at high pressure which concentrates all the heat removed by the evaporator. --------- Personally, I'm more interested in Air cycle system refrigeration, than vapor compression. It produces a much greater temperature differential and uses no dangerous, ozone depletion or greenhouse gas. In applications where both poles of the temperature differential can be utilized, it's efficiency is good.
-
The "Ice Bomb" thermal engine
So where exactly do you think the heat removed from the inside of the refrigerator goes? Granted, more efficient compressors may be available than typically found in a domestic refrigerator. The refrigerant still circulates in a loop. A refrigerator is the same as a heat pump. It's job is to move heat from inside the refrigerator to the outside. The heat cannot dissipate to the outside without being elevated in temperature. Since heat only flows from hot to cold. Therefore, the refrigerant gas is compressed. The heat is concentrated so it is warmer than the outside air, that way it will transfer to the outside. The heat from the inside could be transfered to a heat engine just as well in exactly the same way.