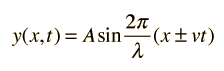-
Posts
792 -
Joined
-
Last visited
-
Days Won
1
Content Type
Profiles
Forums
Events
Everything posted by Butch
-
Intrinsic angular momentum... Sorry, I am trying to get my head around this. By the way I quit smoking almost a month now, quite a feat while delving into quanta. Lol.
-
I believe you, but I don't see it... Are you saying that max amplitude is at either peak, akin to alternating electrical potential? Is that why I was asked about the sine function? All understood, however if the polarity of L does not change wouldn't the amplitude (A) always be positive? Ok... Not all understood, your plot is the tangent... Does this refer to the derivitave of the equation and containment must be applied because of infinity?
-
I am somewhat adept at the maths from the number line to basic calc... Quantum mechanics is new ground for me, I am trying to have a good understanding of the maths, not just rote rehearsel. From what I have studied this is where Schrodinger began, a time dependant traveling wave. The "n" is where my question arises... 2π/y represents a single cycle or an integral number of cycles. Should not "n" be represented by the same integral? Understood, but as I understand it the integral is a full wave. Let me try getting to the point without being humbled traumatically... If n = .5 the result could be a low energy p orbital masked by the s orbital. This p orbital however would allow only a single electron. With my current understanding, I would apply inverse square for the energy of this orbital.
-
Okay, perhaps you could provide some assistance, what does the quantum number relate to, and is it demonstrated in the above equation (I know this is not the Schrodinger equation.)? I am aware of constraints, however it seems that if the quantum number is related to wave function it must also be related to wavelength, where am I getting lost?
-
I have been studying Schrodinger's equation, and I have a question... Given the representation of a moving wave: Eventually the quantum number corresponds to the number of wavelengths in the circuit of the electron in the orbital (discounting spin)? Is my understanding correct?
-
You are pushing my thinking, thank you! There is discussion for this, however I will reserve it for a later time. If we can know the position at t0 and the position at t1 we can construct a vector, we cannot know the actual path. Correct? My question has been answered, there will be more, but I will confine them to new topics. Again, thank you very much... Y'ALL are awesome!
-
The plane perpendicular to the axis of the orbital, this plane is probably not static. In the case of an s orbital of 1h the plane is likely not in play as the nucleus is a single proton. It is my feeling at this time that the electron and proton do not merge... That could however be incorrect. To elaborate I would be treading in a closed thread. You have answered my question, thank you! Can we say then that an electron can be located in the nucleus? There is no trajectory, however there is a vector, correct? ∆p ∆t...
-
How about a current events section in the lounge?
-
Reality is a persistent illusion, so it is with time. Ultimately we must accept the illusion.
-
Perhaps we cross posted... I am not speaking in terms of trajectory or velocity, only probability... It is constantly stated that the probability of an electron in the nucleus is zero, that is quite different than approaches zero... As Strange will agree "Words have meaning". I am, thanks very much to the members of this forum! I do apologise... The "plane of the nucleus", rather than the "orbital plane of the nucleus". The 1s orbital is unique, what of 2s and up?
-
I am not trying to calculate velocity... Yet. I am trying to demonstrate that an electron can pass through the orbital plane of the nucleus, and still have a probability of being in the plane that approaches zero. The nucleus has a center of mass a plane of the nucleus would intersect the center of mass perpendicular to the orbital axis.
-
But the intersection of the orbital with the plane of the nucleus is, as I understand it the probability of an electron being in this plane is zero, would it not be correct to say rather that the probability approaches zero? Although velocity is not well defined, still an electron has a wave function and particle manifestation, so it has velocity. Lol, yes indeed! But that limit approaches zero, which as you know the calculus fish says is much different than zero. Let me be a little clearer the intersection of the orbital with the plane of the nucleus is perhaps an infinite number of points, however the set of points in the orbital set is infinitely greater. Thus the intersection approaches zero. That calculus fish again.
-
.thumb.jpg.cc169fb67f9c969aeace623286b9b5e6.jpg)
Holding down steel plate w/ multiple magnets: Does orientation matter?
Butch replied to Wookbert's topic in Classical Physics
You are correct! It is counter intuitive. -
The electron has velocity. Please elaborate, it will be much appreciated.
-
.thumb.jpg.cc169fb67f9c969aeace623286b9b5e6.jpg)
Holding down steel plate w/ multiple magnets: Does orientation matter?
Butch replied to Wookbert's topic in Classical Physics
The steel would concentrate the magnetic fields, in the second orientation the resultant field would be much weaker than the first and thus would allow for easier movement along any axis. -
.thumb.jpg.cc169fb67f9c969aeace623286b9b5e6.jpg)
generating electricity with fusion explosives
Butch replied to trevorjohnson32's topic in Classical Physics
My idea would be to create a giant fusion reactor at a safe distance from the earth, perhaps on the order of 100,000,000 miles and use collectors to convert the radiant energy into electricity. -
Consider the following: The probability of an electron being at any single point within an orbital domain approaches zero. The probability of an electron being in a defined area of an orbital domain is directly proportional to the dimensions of the defined area and inversely proportional to velocity. The area of an orbital intersection with the plane of the nucleus is a single point, thus the probability of an electron being in the orbital plane approaches zero. Comments?
-
Thank you.
-
I apologise for not making my inquiry clear, however it has festered and come into better focus... Consider the following: Electron A has velocity that produces magnetic moment n1, electron B has velocity that produces magnetic moment n2. Would the force between A and B be greater than that stated in Coulomb's law? If not why? Hmm, it was my understanding that it did, perhaps I should reread? Okay, if it is orbital L would that not affect the force between particles?
-
I am saying that taxes of any kind get passed down to the consumer.
-
How do they relate? If velocity increases magnetic moment, is charge affected?(Lenz's law).
-
https://www.indexmundi.com/g/r.aspx?v=2223
- 277 replies
-
-1
-
Our government is corrupt, unbelievably corrupt, regardless the party... The Bushes are crooks and so are the Clintons. The world wants America back, so do Americans! It is not about party, So please quit blinding yourselves with that game. I did not vote for Trump because he is a republican(he is not), people voted for Trump in the hope that he can break up the game in Washington! Capitol Hill has taken notice and many more heads are going to roll, either by executive action or by the will of the people! Talking party loyalty or social agendas is useless until we get rid of the stench in Washington, then we can look to helping the rest of the world.
-
First we need to drain the swamp Dems and Reps. You should understand that polarization is a very old strategy,... We have the very best healthcare on the planet!
- 277 replies
-
-3
-
There The problem with the "dole" is the government does a poor, but expensive job of administering it. It was much better accomplished when neighbor helped neighbor... Since the creation of the "Great society" it has gotten to the point of neighbor doesn't even know neighbor.