Everything posted by J.C.MacSwell
-
Comparing Corona Virus Success Stories with Abysmal Failures
Canada seems to have done about the same number of tests as the US, despite the US having almost 10X the population. Growth in cases, for both Countries, is still exponential with the US at a higher rate. Canada's numbers doubling every 3 days, the US's in less than 2. Hopefully we see a flattening of the curves soon, or at least signs of it, as isolation measures show their effect.
-
Corona virus general questions mega thread
This seems reasonable if home alone, no one else has been in for some time, and you don't expect anyone to come into your home.
-
Corona virus general questions mega thread
Even if you don't clean your remote and phone (which as CY suggests you can) all else being equal you've reduced your level of exposure. You pay your money, or put in the effort, and you take your chances.
-
Comparing Corona Virus Success Stories with Abysmal Failures
Germany's numbers match up well against pretty much any country, even those on your success list.
-
Corona virus general questions mega thread
You probably are aware of this one already but it's a good starting point. https://en.wikipedia.org/wiki/2019–20_coronavirus_pandemic I've seen some news reports where you could infer that you are roughly 50% more likely to get it if blood type A, than blood type O. Nothing clear why.
-
Corona virus general questions mega thread
Most died of bacterial pneumonia, so better antibiotics would have saved many if available at that time.
-
Corona virus general questions mega thread
Agree. Also, wouldn't it dilute the samples to make false negatives more likely?
-
Corona virus general questions mega thread
Thanks CY. One more: I can understand why older individuals with compromising health issues would be more at risk, but why would the the very young with underdeveloped immune systems not be more at risk than those still young and healthy but with fully developed immune systems? Covid-19 seems to, thankfully, have spared the very young for some reason, compared to other viruses and flus relatively speaking. I believe the Spanish flu was relatively hard on the middle group that normally would be in the optimal range.
-
Corona virus general questions mega thread
What about exposure at a level where you don't "catch" it, or it doesn't take root? Will this have given you at least a head start on reducing susceptibility if exposed to a greater extent later? Or if you do catch it, is a lesser exposure still consistent with a better outcome? (all other factors being equal) How about exposure to the dead remnants of the virus, say from the a surface that has been exposed to sunlight? Any potential for positive effect going forward? I'm sure I have some misconceptions leading to these questions and would be interested in where they are.
-
Corona virus general questions mega thread
Thanks SJ, CY.
-
Corona virus general questions mega thread
Assuming you have caught a lesser coronavirus or cold, are you more or less likely to catch Covid-19 if exposed? Same/different for other virus's? Bacterias? Fungi? Does the timing matter? Is there a "crowding out" effect in place sometimes vs a "rundown" effect other times?
-
Corona virus general questions mega thread
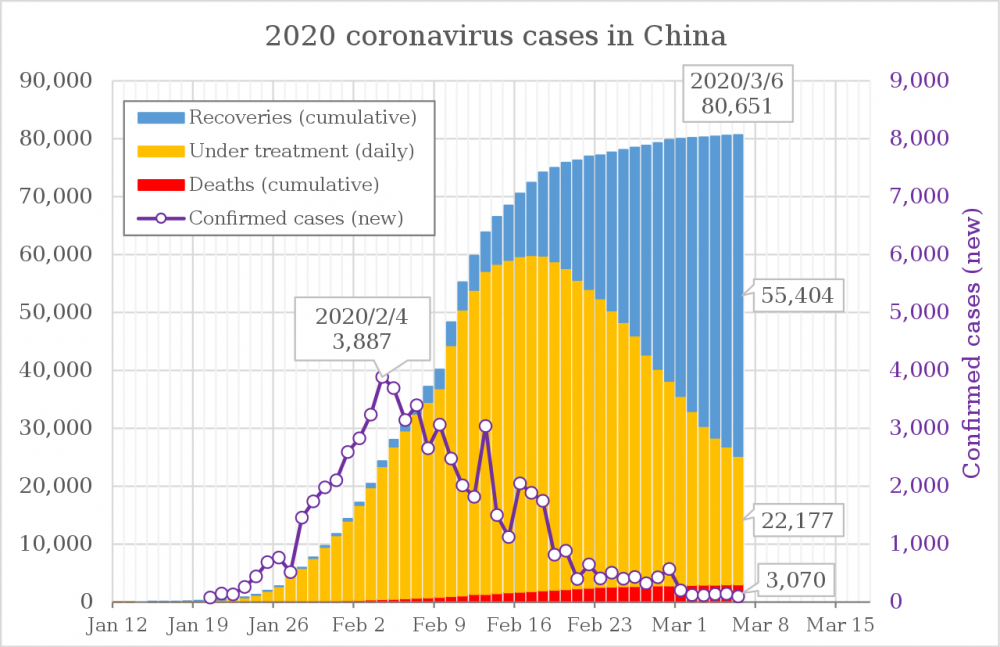
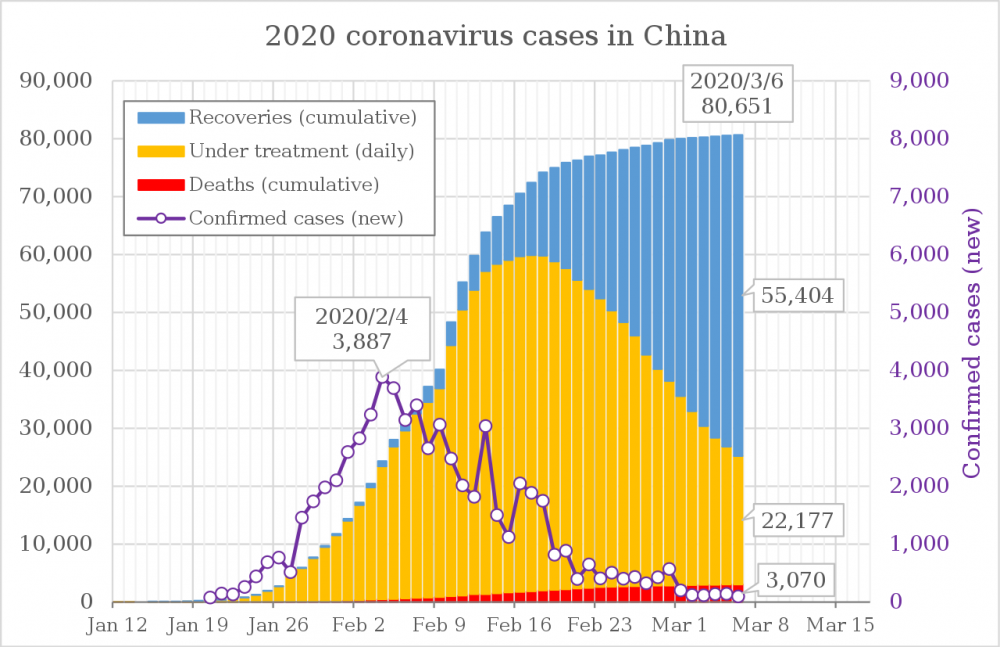
Seems like China has the Covid 19 virus down to a 0.1%/day growth rate. (Though that is based on total cases including those recovered, if I read it correctly): 80,651(+0.1%) https://en.wikipedia.org/wiki/2019–20_coronavirus_outbreak_in_mainland_China
-
Debt-free College (Yang's Plan) - Split
Where did he say that? And are you suggesting the purposes of higher education, especially with regard to government funding, don't include preparing for careers?
-
Debt-free College (Yang's Plan) - Split
My bad, but you have a (much smaller) typo as well. $1,000/month. 12K/year.
-
Debt-free College (Yang's Plan) - Split
I think he is actually very well informed and has good incites. Would you prefer his thoughts to be finalized but off base? Do you think that Biden, Sanders, or (LOL, can he find Kansas City on a map?) Trump are better informed on the subject circa 2020? How about Buttigieg? Warren obviously has a background on this...but while she takes a narrative, always, Yang looks to evaluate the problem. He doesn't expect or want you to fall in line, never mind berate your ideas if they don't align. I know it's a different approach...not assuming you're right about everything but still willing to submit your ideas to scrutiny and admitting pros and cons of them. I get the thought (not the reality, IMO) that he's not ready yet...that the UBI at $12,000/month is to high (IMO), and that at times he's spitballin (in an honest manner) toward solutions, but he's the best candidate, by a considerable margin, out there (obviously IMO).
-
Debt-free College (Yang's Plan) - Split
Many of his policy proposals are simply outlining his thoughts. I think this is, or should be, a good thing in many cases, where he doesn't expect to dictate the details. I know I'm in the OP, but I didn't start this thread. (I do think he is the most capable candidate, including on this subject, in the actual POTUS role to address it) +1 for the post +2 if I could, for checking out his website
-
Debt-free College (Yang's Plan) - Split
You're admonishment is longer, and just as off topic.
-
Debt-free College (Yang's Plan) - Split
Quite possibly, but how would we know that? If it's more than a comment we can look at opening a thread. If it's because, say, Yang doesn't wear ties that would hardly be worthwhile. https://www.yang2020.com/policies/student-loan-debt/
-
What is Space made of?
Congratulations Studiot...on your 11111th post!
-
"Wait a carrot-picking minute...
Not sure, but I've been told on occasion I have some on my shoulder. I just get laughed at when I try to find out what it is.
-
What is the Purpose of Life ?
I just try to be a better person today than I was yesterday. That's the key to my success... ...setting the bar low
-
Why does Gorilla have small penis compared to humans?
I wonder if the wearing of protective clothing, or having the option of wearing clothing may have something to do with it? The gorilla's might be more vulnerable in that respect.
-
Harnessing the power of hurricanes
Marginally, but so would much cheaper floating obstacles of similar size (maybe slightly bigger) When I say much cheaper...I mean very expensive to have any measurable affect.
-
Harnessing the power of hurricanes
Interesting (though no doubt expensive) idea as you might match the power of the mix to maintain the grid power the field normally generates, effectively at least for smaller hurricanes.
-
Harnessing the power of hurricanes
"Hurricane mode" is feathered (not turning and not producing power) so as not to expose the blades to 8 times times the energy going through the disc for every doubling of wind speed. From your quote: "Sandy’s wind speeds dropped below hurricane status just before landfall, but the Infigen turbines still withstood sustained winds of 65 mph [105 kph] or so, with gusts reaching much higher, as the center of Sandy passed right over them. The turbines were undamaged, said Matthew McGowan of Infigen, and were soon generating 1.5 megawatts of electricity again." As impressive as this might sound, they were feathered/not turning at the 65mph quoted...which is barely over 1/5 of the wind energy going through the disc than it would be at the 110mph wind speed claimed by the writer of your original post for the Cuban turbines.