

UC
-
Posts
547 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by UC
-
-
/me hands ydoaPs a copy of "The Hungry Caterpillar".
That book may be overkill in countering this dissertation, but it's the only suitable biology book I had on hand.
I liked that book as a kid

On another note, I find the description from the "University's" site exceedingly pitiful- "Patriot course books are carefully selected from Biblically conservative, professional authors who have written on a focused subject. Courses present and expound on the absolute truth of the Bible - not humanistic reasoning."
0 -
You could get some luminal, put it in a beaker and pour in some hydrogen peroxide. It will glow a bright blue for several hours.
This is from here: http://www.ehow.com/how_4859147_make-glowinthedark-liquid.html
Pour 1 liter of distilled water in a bowl with one bottle of hydrogen peroxide.
Pour another liter of distilled water into a second large bowl. Add 4 grams of sodium carbonate to it as well as .4 grams of copper sulfate pentahydrate.
Add .2 grams of luminol to your second mixing bowl.
Combine the two bowls and you will immediately see your glow in the dark liquid.
I have not tested this personally so do it at your own risk.
luminol has a glow lifetime that's on the order of seconds, not hours.
0 -
Always check the synthetic literature first- http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv2p0434
I suggest you take the current product and treat it as in the second paragraph and on, scaling portions for your amount of product.
It looks to be rather conditions-sensitive. You may want to re-run the procedure using the recommendations there in order to minimize difficult-to-seperate side products.
0 -
Look up a 3-center, 4 electron bond. I don't see why the mechanism couldn't use that as a high-energy intermediate with sp2 hybridization of the central carbon.
0 -
Hydrolysis of what? Esters?
Draw out the mechanism and look for a step that might involve an acid or base.
Lewis acids?
Look at the reactants and products with regards to Le Chatlier's principle.
0 -
Just a heads up: Being a ChemE is a lot of math and not a lot of chemistry. You do some chem early on, but the higher level material is all fluid dynamics, properties of materials, etc.
Basically, the job description is to design and maintain equipment used in chemicals processing. This could be an oil company, a smaller company that produces inks or pharmaceuticals, or even a food production plant.
If you like tinkering with flasks and reactions, it's not the place for you.
0 -
A volatile solid, which sublimes at room temperature, this compound has a characteristic odour similar to that of ozone. It is used widely in organic synthesis, and as a staining agent. It was also used to confirm the structure of buckminsterfullerene.
Too many hints. It's OsO4. If you can smell it, you're not using enough safety equipment and should head for a hospital, as it can cause pulmonary edema and death below these concentrations. For the same reason it can stain tissue specimens, the fumes will damage your eyes and can cause blindness.
Next!
I am a very strongly colored kind of "salt", with an unconventional anion. I have been known for a very long time in solution, but have only recently been isolated as a solid using special ligands. You might say that the cations are as tightly bound as if they were in a grave. What am I?
0 -
5-aminotetrazole, [ce]CH3N5[/ce]?
Seems to fit the description quite well... not sure how stable it is.
Ok, I cheated a bit... I reverse engineered the solution using the percentage to find out how many C and H there were (using the assumption that C and H were the only other elements). I had never heard of the component until 2 minutes ago.
Next one:
I'm the glue of life, a heavily branched polymer, but not a sugar polymer. Without me, trees would fall.
Damnit, yep.
0 -
However quantum mechanics has demonstrated that the universe acts differently when we're looking as opposed to when we let it be.
That depends on how you're observing the system. In this case, intercepting vibrational waves outside of the area of the event and in a time after the event has occured is highly unlikely to alter the state of the tree having made a sound. When you have to directly perturb the system to make a measurement, things get ugly.
The real question is "If a quantum mechanical tree falls in a one-dimensional forest and no physicist is around to calculate the expectation values, does anyone care?"
0 -
The problem here is that you are comparing two entirely different reactions.
When you use HCl, you are basically doing chemistry with the H+ ion present. The Cl- is pretty much inert. Look up an activity series. HCl will attack anything above hydrogen, including, but not limited to, lead, tin, zinc, aluminum, magnesium, sodium, etc. Lead is a bit fussy since lead chloride is insoluble and will form a protective coat that stops the reaction from proceeding, but it will react on the surface and heating helps drive it further. Copper is below hydrogen and so is not attacked.
When you use dilute nitric acid, the reactions are identical, but when you use concentrated nitric acid, you are no longer doing chemistry with that H+. Instead, you are utilizing the nitrate portion, which is a powerful oxidizing agent when in high concentration. The nitrate portion is reduced to NO2 gas and the metal in question is oxidized. This reaction allows concentrated nitric acid to also attack metals that are "below" hydrogen on the activity series (to a point). Mercury, copper, and silver are all readily attacked by this reaction.
The only reason that aluminum escapes oxidation is a total fluke. Aluminum has a high propensity to form a protective layer of aluminum oxide (corundum, the stuff rubies and sapphires are made of) and both in everyday air and under the strongly oxidizing conditions of concentrated nitric acid, this is exactly what happens. In more dilute solutions, the acidity would be able to dissolve this layer as aluminum nitrate and then the acid could attack as usual.
Magnesium is fairly close in reactivity to aluminum, but doesn't form such an oxide layer. It is rapidly attacked by strong nitric acid, as aluminum would be if it weren't for the pesky oxide layer. Of course, the oxide layer is what makes aluminum so great as a building material. Without it, anything made of aluminum would crumble in a few months at most from oxidation by air.
0 -
with an empirical formula of [ce]C2H[/ce], this molecule is highly reactive. It has been identified in the atmosphere of titan and in some interstellar clouds. it is closely related to the monomer for rubber making.
Diacetylene. http://en.wikipedia.org/wiki/Diacetylene
Next!
With 82.33% nitrogen by weight, I narrowly beats out ammonia itself (82.24%) for nitrogen content. I carry at least some aromaticity in my heterocyclic ring, and I'm known to have an unstable personality when dry. What am I?
0 -
Just adjust the pH with ammonium hydroxide. You'd just be mixing the two to make a different ammonium phosphate anyway, which would be making more work for yourself to do seperately.
0 -
Dowsing and divining have NOT been PROVEN to be hoaxes or fake. To be fair the most you can say is that Modern Science cannot explain this phenomena. There are MANY people who swear by it's working, where dowsers have found water when modern surveying equipment has failed.
Your claim that " Even the most "accomplished" dowsers fail to achieve anything more than chance rates in a properly controlled experimental setup.' is simply made up off the top of your head and is Not a provable fact.
All expert dowsers the world over have Not been tested. You have no way of knowing if the dowsers tested were really "accomplished" or not. This is just another tragic case of science bashing something it cannot explain or understand. True science would not presume to make the statements you have made about divining.
To be fair you can say "It hasn't been proven yet or science can find no proof to explain it" but you cannot say it is always a hoax since science cannot prove this.
Substitute every word about dowsing or divining for something about homeopathy, telephone psychics, prayer, etc.
0 -
Could urea possibly hydrolize at room temperature very slowly?
Provided strong base, of course. Heat just makes it go much faster. Even cold ammonium nitrate solution and cold sodium hydroxide solution would have stunk like ammonia as soon as mixed together
0 -
Start: [math] dU=TdS-PdV [/math]
Divide across by dV and hold T constant.
[math] (\frac{\partial U}{\partial V})_T=T(\frac{\partial S}{\partial V})_T-P [/math]
Use the maxwell relation [math] (\frac{\partial P}{\partial T})_V=(\frac{\partial S}{\partial V})_T [/math] to get:
[math] (\frac{\partial U}{\partial V})_T=T(\frac{\partial P}{\partial T})_V-P [/math]
Starting from [math] PV=nRT [/math], take the differential form and hold V constant:
[math] d(PV)=d(nRT) \rightarrow VdP=nRdT \rightarrow (\frac{\partial P}{\partial T})_V=\frac{nR}{V} [/math]
Substitute that in to get:
[math] (\frac{\partial U}{\partial V})_T=\frac{nRT}{V}-P [/math]
Simple rearrangement of the ideal gas law gives the value of the right side of that equation:
[math] PV=nRT \rightarrow P=\frac{nRT}{V} \rightarrow 0=\frac{nRT}{V}-P [/math]
So, overall:
[math] (\frac{\partial U}{\partial V})_T=0 [/math] [Valid only for ideal gases]
0 -
MS uses incredibly tiny amounts of the substances involved, so there's no issue. The worst that happens is that the sample decomposes or fragments.
The ionization can occur by a large number of processes, some of which are very gentle to the molecules (Things like MALDI work on giant proteins without destroying them). The most common is probably electron impact, which tends to fragment the parent ion to a large extent, allowing structural information to be determined.
It is possible to run a negative ion MS, but that requires atoms like halogens to be present.
m/z analyzers vary from standard quadrupoles, magnetic sector analyzers, time-of-flight (TOF) tubes, and the holy grail being ion cyclotron resonance. Quadroples are quick-and-dirty and work well enough on small molecules.
0 -
How about plutonium with a -94 charge on it?
wouldn't a naked [ce] ^{62}Ni [/ce] nucleus do better? Or would increasing the atomic number merely start to give diminishing returns?
0 -
Oh, I forgot to mention, I did another test for ammonium ions, and it was positive. I just reacted it with sodium hydroxide and it bubbled ammonia gas which formed a white ammonium chloride mist with hydrogen chloride gas.
Thanks for the info, guys.
OK, I'm pretty sure it's urea. What does urea dissociate to in water? I've reacted the urea with excess sodium hydroxide and boiled off all the liquids, and have gotten a yellowish white solid. The yellow may, however, be due to the sodium hydroxide reacting with an unfortunate fly that I found had landed in it. Is this new salt possibly sodium cyanate?
Can you smell ammonia coming off of a cold solution of the unknown salt mixed with a little NaOH, or do you have to heat to smell it? It hydrolyzes just like any other amide, but that won't happen with cold, dilute base. The condensation to cyanuric acid only occurs when molten, so you solid is almost surely just carbonate and leftover hydroxide.
0 -
It's urea. The insoluble white stuff is cyanuric acid, melamine, any of various intermediates between the two, or some ungodly polymeric crud. The fumes while heating were probably mostly ammonia, but if you applied a lot of heat, you could be getting isocyanic acid (HNCO, not cyanide) as well.
It's being used in cold packs because it works about as well as ammonium nitrate and is less hazardous (and can't be used for pyro).
0 -
As already stated you will have a rough time getting a decent seperation of this mixture. As long as you are not hell bent on using distillation as the means of seperation then it will be a walk in the park.
Sodium Hydrosulfite(Sodium Bisulfite) will form the sulfonate adduct with the acetone forming a solid precipitate allowing you to filter and redistill the resulting acetone free Ethanol. Keep in mind I said Bisulfite and not Bisulfate.
Hydrosulfite (dithionite) is not the same as bisulfite. Perhaps you are thinking of metabisulfite, which is a dehydrated form of bisulfite.
The problem is that we're dealing with 60% acetone in solution, and producing the bisulfite adduct requires adding water. If you use enough water that the complex is filterable, you are going to be left with very dilute ethanol solution, and the distillation thereof will leave you with the azeotrope at best.
http://www.chemeng.ed.ac.uk/~jwp/procalcs/procalcs/mixtures/nonideal/slides/data/acet-eth.gif
That chart shows no azeotrope or funny business of any kind. Plain old fractionation should yield pure ethanol and pure acetone. Consider using a better column (is yours insulated and is it packed or a vigreux?) or making sure your thermometer is properly calibrated.
0 -
Thank you so much for your answer. I understand PKa now but I have a problem.
In an amino acid there is only one molecule of SH. How can there be a large group SH molecules in one amino acid. Is the question talking about like one amino acid or mole of amino acid. I know I'm not understanding something obvious but please help
Thank you!!
Oh, they just mean an arbitrary solution containing a bunch of it. It's just the convention to speak about it as if it's one thing when discussing functional groups and pKa, etc.
0 -
A Pka value is not a pH value. Both, however are equal to [ce] - log_{10}[X] [/ce] where X is the Ka for pKa and X is the hydrogen ion concentration for pH.
Here is the Henderson-Hasselbalch Equation:
[math] pH=pKa+ log_{10}([A^-]/[HA])[/math]
In this problem, we need to treat the -SH group as a weak acid that can lose it's proton. The anion form is represented by [ce] [A^-] [/ce] and the sulfhydyl is represented by [ce] [HA] [/ce]. Technically, the brackets indicate concentration, but since you're only interested in the ratio, it's irrelevant whether the solution is 1M, 10M or 0.005M.
They've given you the pH and pKa values to plug in. Then you just need to remove the [ce] log_{10} [/ce] part of the term to get the ratio of anion to sulfhydryl.
I don't understand what you mean by "how can an equilibrium reaction occur?"
[ce] R-SH <-> R-S^- + H^+ [/ce] is an equilibrium reaction. The equilibrium constant represents the tendency toward the right or left of that equilibrium arrow.
0 -
Here's a "snapshot" of one of the resonance forms of the ion. As you can see, there is a quaternary nitrogen present. If you use Huckel's rule, I believe that you will find this is an aromatic ring, and the positive charge is delocalized for that reason.
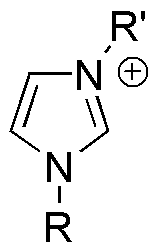 0
0 -
Whoop de freaking doo. Welcome to all of chemistry. Would you like us to "beware" the other 6 million compounds that are oxidizing agents? What about oxygen itself? And what about reducing agents that can combust violently in the right conditions? Don't you live in a house? That's made of wood! It's HIGHLY flammable!
Humanity needs one big warning label for absolutely everything: "Don't be an idiot." and then we let the Darwin awards weed themselves out.
What about scissors? I could hurt myself with those too. Or breathing. The oxidative damage will eventually kill me. Driving a car is pretty suicidal too, if you think about it.
-3

White Powder Gold
in Speculations
Posted
Is the name Vlad perhaps familiar to you?
http://www.sciencemadness.org/talk/viewthread.php?tid=4700