
John Cuthber
-
Joined
-
Last visited
Posts posted by John Cuthber
-
-
-
On 3/19/2017 at 11:04 PM, CharonY said: Possible and rather typical for what effectively is fusion food (but less pretentious). Incidentally, food is for me one of the best arguments for immigration.
I must have been busy 9 years ago so I missed the opportunity to post this.
https://www.youtube.com/watch?v=YaGdwfykYGY -
Could someone do me a favour please?
Tell Trump that North Sea oil has pretty much run out, so there's no point in invading the UK.
Thanks.
(on behalf of about 70 million of us)
And , now for the science part...16 hours ago, swansont said: the oil companies are less than thrilled with this. Heavy crude (and with contaminants like sulphur, so it’s “sour”) is more expensive to refine. It’s used for diesel, not gasoline, but having more on the market just drives prices down.
When North sea oil came on stream, Venezuelan oil actually became more valuable.
As you say, Venezuelan crude is heavy.
On the other hand, North Sea crude is much lighter.
By blending them, the oil companies could use the mixture in their refineries that were designed for use on Arabian oil which is intermediate between the two. -
Edited by John Cuthber
2 hours ago, Externet said: Nobody can ruin Venezuela more than it already is and has been for decades.
If I remember well, around 1968, petroleum was discovered there. That is 45 years x 360 days = 16200 days x 1 million barrels per day exported petroleum x dollars per barrel = the calculator just crapped.
Where that country used such huuuge amount of wealth when they have no toilet paper to wipe their butts ? The same typical reason; their politicians ransacking all the time. It is not the removed president; it is all of them since ~1970.
Let the justice decide if jailing their master is right or wrong. If the reason is drug trafficking, let it be.
It's clear that Venezuela has been gravely mismanaged for decades.
And now, they will get "run" by a man who managed to bankrupt six casinos and thinks you can drop drug prices by 800%.
I guess the good news is that the people are used to being shafted.
It's also fair to say that, if Trump's claims about drugs are honest, the USA will no longer have a drug problem.
Is anyone taking bets on that ? -
-
-
23 hours ago, TheVat said: The writer is advancing the opinion that overuse of a slang or diminutive form of a word can debase proper usage. We all pushed back and pointed out how the word cello had long ago become accepted, and the usage should be seen as a normal part of the evolution of language. The same with pianoforte becoming piano a couple centuries ago.
Yes, but "So improper wording rules by accepting the wrong slang." doesn't seem to parse in English.
Did you not see the irony? -
-
On 2/20/2025 at 2:17 PM, Externet said: Good morning mister president.
What is your vision for Gaza and Greenland belonging to the U.S. ? Are them to become others Puerto Rico or assets to trade for else ? In four years when you be replaced by your successor; what reversals will you hate the most ? By the way, is Puerto Rico on your sight ?
Apparently the "Trump said he "talked to the president of Puerto Rico"" story is false but the
Trump's remarks to the 2017 Values Voters Summit, at which he misspoke, saying he had met with the "President of the Virgin Islands".
story is true... -
On 9/25/2025 at 4:16 AM, Linkey said: As far as I can see, if we compare USA with Europe, the latter looks like USSR: it has more equality, but less freedom. The laws control everything in Europe; for example, cucumbers for sale must be of a strictly defined shape.
Comparing things to an institution which has not existed for about a third of a century is... odd.
On December 25, 1991, the Soviet hammer and sickle flag lowered for the last time over the Kremlin, thereafter replaced by the Russian tricolor.
I own a 3 necked flask.
It's just a bit of glass, but it is banned in Texas.
You can sell any cucumber you like in Europe, provided that it's fit to eat, or clearly marked as not being so.
You seem to have fundamentally failed to grasp the difference between US and UK (and, to an extent, the rest of Europe's) law.
Americans often point out that the UK has no "bill of rights".
That is true.
We don't need one.
We effectively have a "bill or wrongs".
We have a long list of laws which tell you what you are forbidden to do (and owning a gun is not on that list, no matter what Faux news told you)
Anything which is not forbidden is permitted by default.
Part of the reason that many UK houses are relatively small is that they are old.
Another is that they are not made of cardboard. -
-
-
This is a 3 page thread that should have been 2 posts.
On 8/12/2025 at 12:50 AM, MJ kihara said: My question is simple can michelson-morley type experiment be able to detect a particle with an energy of 1×10−64 J or a stream of such particles @ 1×10−64 J⋅Hz−1
Discuss....My intake is NO.
On 8/12/2025 at 2:01 AM, swansont said: They’d be massless (or some new particle with a vanishingly small mass)
So, what is the wavelength of such a photon?
hc/E is around 2 x 10^38 m
A LY is 10^16 m, so 2 x 10^22 LY
To get destructive interference you need a path length difference of half a wavelength. Seeing as this is much, much longer than the size of the visible universe, I’m guessing no. Plus the time it would take to run the experiment.
The contrast you get with a reasonable size interferometer would be vanishingly small
Not sure of your units. J/Hz? Did you mean J/s?
Why don't we all stop here, and pretend that it was?
The question (in spite of a typo) was fully answered in the first reply. -
-
I never studied ant anatomy, or really studied that of humans.
But I know that we can burp, and I don't see why ants would not be able to do so.
Exploding ants seems unlikely.
However, if they eat a significant amount of sodium bicarbonate, that will throw their electrolyte balance out of kilter.
Also, in many countries, compounding your own unlicensed insecticides is illegal. -
-
I'm not sure there's a useful definition of "Okay" which includes the situation in the USA.
I note with some amusement that Trump is "only" a US citizen by birthright.
It would be perfectly possible to legally remove his citizenship under the laws he's planning to introduce.
Seems fair.
Maybe deport him to an El Salvadoran jail? -
This map indicates in green, the states where it is forbidden to deprive a person of statehood if that person is a child.
And it shows an uncivilised country in purple.
https://en.wikipedia.org/wiki/Convention_on_the_Rights_of_the_Child#/media/File:Convention_on_the_Rights_of_the_Child.svg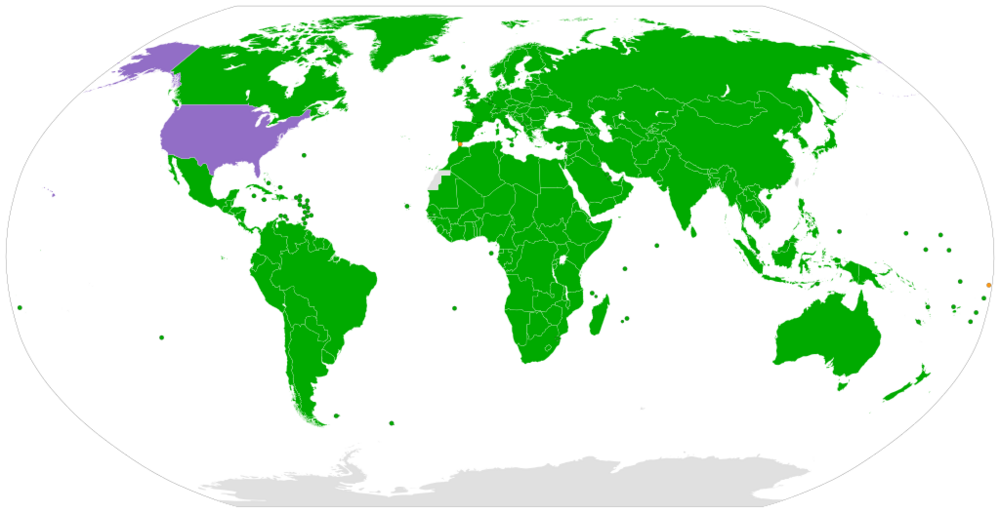
-
7 hours ago, exchemist said: The first route would seem to suggest esterification, i.e. with the hydroxyl groups of the glucose units making up the maltodextrin polymer.
It may suggest that to you, but not to me.
I know that maltodextrin is used as a sorbent
I'd expect any acetate of maltodextrin to behave a bit like either
https://en.wikipedia.org/wiki/Sucrose_octaacetate
which is very bitter
Or
https://en.wikipedia.org/wiki/Cellulose_acetate
Which is tasteless.1 hour ago, toucana said: "To make dry vinegar which you may carry in your pocket, you shall take blades of green corn, either wheat or rye, and beat it in a mortar with the strongest vinegar you can get till it come to a paste; then roll it into little balls, and dry it in the sun till it be very hard; when you have an occasion to use it, cut a little piece thereof and dissolve it in wine, and it will make a strong vinegar."
That's interesting.
I wonder if it was chemistry or microbiology.
If you add an inoculum of acetic acid bacteria to wine, you will get vinegar.
It does seem redundant to have a solid form of vinegar in the same cruet as the liquid- but it's not impossible.
It's possible that the filling for the "third pot in the set" was left to the choice of the household (or their butler). -
On 6/5/2025 at 2:01 PM, exchemist said: I must say I have never heard of such a thing.
I don't know if it was in common use. I have seen it listed as a flavouring.
But the point is that it wasn't hard to prove that it was notOn 6/5/2025 at 10:28 AM, exchemist said: utter nonsense.
A spot of googling would have told you that, and I think you owe the OP an apology.
-
3 hours ago, exchemist said: “Vinegar powder”? That must be utter nonsense.
-
-
-
Messages to the president...
in Politics
Mr President; someone has suggested a name for the new Ballroom.
https://en.wikipedia.org/wiki/Orangery