-
Posts
456 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by Tom Booth
-
-
8 minutes ago, exchemist said:
That's ballocks. It is an ideal theoretical engine cycle, with constant temperature heat input and output. The environment has nothing to do with it.
I thought the equation was based on the hot and cold "reservoirs" The engine sits between.
Is that not correct?
like a water wheel between an elevated body of water and a lower body of water.
the Carnot "efficiency" is a measure of the "height of the fall" not the actual mechanical efficiency of the water mill (or engine) to convert "available" energy. It is the temperature difference, not how well the engine actually utilized that temperature difference. The actual engine has nothing whatsoever to do with it.
0 -
2 minutes ago, Ghideon said:
Sharing is ok with me but note that my question was a rethorical. Elaborating may take the thread off topic but I’ll clarify if you wish.
Well OK. Rhetorical.
So you thought I would say "Oh no, that can't work!!!" or some such response?
Hey, my theory is perfectly falsifiable by your method. I'm very grateful for the suggestion. If there is a way to DISPROVE it, conclusively, then I can put all this behind me and get on with the rest of my life.
I have no desire to continue wasting time and money on nonsense.
0 -
-
4 minutes ago, exchemist said:
Don't be ridiculous. This is a total ranting rhetorical muddle as usual and contains a stupid straw man. There is no such thing as a "Carnot engine". You have made that up.
There is a Carnot cycle, which, as several people have told you several times, is a theoretical optimum heat engine cycle whose thermal efficiency, according to the theory of thermodynamics, no real engine can exceed.
The theory of the Carnot cycle would thus obviously be falsified if someone were to produce a heat engine exceeding Carnot cycle efficiency. So it is - obviously- a falsifiable theory, in Popper's terms.
The "Carnot efficiency" relates to the environment, not the engine. So it is not even theoretically possible to build or "produce an engine" that exceeds the result of calculations unrated to the performance of the engine.
Well, with a possible exception.
If the engine is also capable of changing the surrounding environment.
2 minutes ago, studiot said:Might that be the line at the end, the only line in the post with a question mark at the end.
I think I did answer that. Your usual rabid impatience resulted in us cross posting. I was still reading through your post and editing, apparently. It happens.
0 -
I posted my response just a few minutes ago. Studiot.
11 minutes ago, sethoflagos said:Off the shelf for sure. Then we have repeatability by other experimenters.
A certain load or drag on a flywheel is not repeatable?
I think both measurements are VERY significant and easily repeatable. Load and no load.
The question relates to work output being correlated with waste heat output. The reciprocal relationship could be plotted on a graph. See how waste heat output correlates with work output etc. Vital information.
0 -
48 minutes ago, sethoflagos said:
Any small known weight sufficient to turn the engine but not too fast.
That should work.
It must be an operable engine. We have no interest in performance data for ruined engines.
Something like that.
If you can perform a second experiment experiment with the displacer linkage disconnected (if this is possible without breaking the engine) then that would be useful too.
OK,
The only possible bone I have to pick is that the conditions I observed where an apparent refrigeration effect took place was with the engine laboring under what I would consider a fairly substantial load. The engine was not ruined until after some extended run time. I think any other substantial load that does not bring the engine to a complete stop would be suitable and cause no harm to the engine. A sime brake of some kind on the flywheel for example, commonly used to test engines. I think that additional data would be important.
Any particular preference as far as the type of engine? Off the shelf, or my own "customized" version generally used for experiments?
14 minutes ago, studiot said:Sigh.
You went to the bother of quoting my post.
And got me all excited.
Thinks : "Perhaps he has bothered to read it."
But No.
Another gargantuan post containing everything but an answer to a simple question.
Thank you for wasting my time yet again.
I am now formally reporting this nonsensical saga.
Wow.
What question exactly did I miss?
0 -
1 hour ago, studiot said:
Please note this thread is allegedly about Carnot, not Stirling.
I do not wish to make a formal off topic complaint which might lead to its closure but I would like to reiterate that I think you still have not understood the true meaning of a hot source or a cold sink (since you seem to wish to use those terms).
I have offered you a formal definition and a block diagram in order to help as proper understanding stands at the very beginning of any analysis. In particular what heat gains and losses occur and where, how and why they occur.
This understanding is also essential to sensible and efficient experimentation technique..
It is so important that perhaps discussing this one subject merits a thread of its own. What do you think ?
Well, there is a guy named Karl Popper, as you probably already know. Falsifiability is mentioned in the rules here under this "speculations" heading.
Arguably, an actual Carnot engine does not meet that criteria of falsifiability, would you agree?
Quick definition via Google: https://www.simplypsychology.org/Karl-Popper.html#:~:text=The Falsification Principle%2C proposed by,by observing a black swan.
QuoteThe Falsification Principle, proposed by Karl Popper, is a way of demarcating science from non-science. It suggests that for a theory to be considered scientific it must be able to be tested and conceivably proven false.
Does anyone have a genuine Carnot engine we can test to prove empirically by experiment that it is actually, ... well, ANYTHING?
How about our perfect hot and cold reservoirs? Is there anything about them we could actually run some kind of experiment on? Use to run any kind of engine?
How about the various thermodynamic processes? Isothermal, Adiabatic etc.
Can we test an engine that sports true Isothermal expansion?
Don't get me started on "Entropy".
Allegedly, all these things constitute "established science", so I'm told.
Established when? Where? How? By whom?
What procedure was used?
Measurements? Actual Data?
I see one thing at least that can be tested in all this; the presence or absence of waste heat leaving a real engine which the Carnot efficiency limit equation suggests is the reciprocal of the work output.
We cannot test the heat input, output, work or whatever of a Carnot engine for any sort of comparison whatsoever, but there is the Axiom, the formula from which it is possible to calculate the maximum efficiency and therefore the minimum reciprocal of efficiency, the waste heat.
Can we measure the waste heat output of some real engine?
Personally, I don't see why not.
Certainly the simple actual presence or absence of any waste heat could be determined if not the actual percentages with exactitude.
As I understand it, there absolutely cannot be < 0 (less than zero).
To disprove the Carnot efficiency equation however, (as currently interpreted) we actually only need to find < 80% of the heat input at the output.
I've basically been told that my measurement of what seems like very near ZERO heat output from my Stirling engine simply means that the heat input is zero. 4 X 0 = 0 so we can all go home satisfied.
Is that really reasonable? Is that argument sufficient? Everybody's happy with that?
Do we need a new thread to define source and sink?
Personally I think everybody has those concepts under their belt, but if you feel they, or something related needs clarification, I have no objections. Agreement regarding terminology is certainly a necessity as a basis for communication.
0 -
1 hour ago, swansont said:
And you can post video as long as the discussion can proceed without having to view it. IOW, as long as the lengthy explanation is there.
Sounds reasonable. In this case some explanation is necessary because it is just a short 2 minute or so segment of a rather lengthy occurrence. The video only shows me prying the engine loose from the ice one time, but this re-freezing of the ice kept happening over and over every time I placed the engine back onto the ice and started it running again. I'd come back ten or fifteen minutes later and it would be stuck again.
After it happened two or three times I got my camera and took the following video, just on the chance it happened again. It did. And then again when I was uploading the video. After that I just let it run and went to bed because I was tired and it was late and I wanted to see what would happen.
When I woke up the engine had stopped and the ice had melted. I couldn't use the engine after that because it had been ground down too much. It apparently ran until it lost compression and then stopped.
So I couldn't determine if it stopped because the ice melted and it lost power, or did it stop because of loosing compression due to the grinding compound.
All I knew for sure is it was ruined, had lost compression, the seal around the piston leaked too much air. and it could no longer be started.
I wouldn't have thought much about it, or I would say, the significance of it seemed greater when I saw an additional video where it appears that a similar low temperature Stirling got stuck to a block of ice in open air, that is, no insulation around anything.
He stated in the video that the engine runs faster and faster as it gets colder and colder, then he picks up the engine and the ice comes up with it apparently stuck like mine was. A rather large heavy block of ice.
In the beginning of the video the engine is constantly sliding to the side almost falling off the wet block of ice but then gradually the engine becomes stable and doesn't slide around any more gets stuck to the ice like mine did is how it looked to me.
If that is enough explanation I can post that video also?
https://youtu.be/L6Jmdve1JK8?list=PLpx2INw8sRqqVTK-hBBerAnBsnwr82var
I know, an alternative explanation is that the wet melting ice stuck on the bottom of the engine like a wet suction cup. But after experiencing the same sort of thing I would have to say it was actually frozen.
At first the engine is sliding around and he has to keep repositioning it on the ice.
Well maybe the room he was in was very cold, below freezing. I don't know, but I know what it looks like. Like my engine, his stopped sliding around and got stuck when the engine ran for a few minutes and the bottom of the engine got cold.
The engines had to be at least reducing the amount of heat reaching the ice for it to have a chance to re-freeze itself from within.
That is what those flow charts show right?
Some heat converted to work so less heat reaches the ice.
But would just a little less heat allow the ice to refreeze like that?
Seems like it would take a whole lot less heat IMO like less than no heat, like maybe putting the ice back in the ice box for a while.
Maybe the guy was using dry ice?
I don't think the engine would slide around like that on dry ice.
 1 hour ago, sethoflagos said:
1 hour ago, sethoflagos said:The only load on the machine is frictional losses, which should be very small and most of those are retained within the system. If waste heat is actually 4 times the work output (it will be a little more), simply ask yourself what 4*0 is equal to.
It might be helpful to know what these no-load losses are:
If you are able to secure a cotton bobbin squarely and centrally on the flywheel and a small weight to the free end of the cotton you can time how long it takes for the weight to drop through say 3 feet. Try and find a weight that's large enough to turn the machine, but small enough to take at least 10 seconds to cover the distance (for timing accuracy). Repeat until your results are consistent and you have at least 10 timings. Then post the timings here along with the distance of travel, size of weight used and the diameter of the bobbin would be interesting as well.
I would assume a weight that was an even metric weight like 10 grams might be helpful?
The only problem is that directly under the center of the flywheel is the engine. Maybe turn it upside down.
Are you interested in the piston being extremely tight and gummed up with grinding compound paste or just an ordinary off the shelf engine. Or both or what, and why? Just out of curiosity.
Calculating frictional loses, no load work involved in just turning the engine. Something like that?
Here is a screenshot from the video at the moment he picks up the engine and the block of ice comes up with it.
 0
0 -
41 minutes ago, sethoflagos said:
A good and important point @Ghideon
An ice block fresh out of the freezer should be oto -18 oC (0 oF) throughout. Even it has been left a while, the surface may well be at freezing point, but the bulk of the inside may be considerably colder.
When it is placed under the cold plate of a Stirling engine, it is effectively insulated from ambient air while it continues to lose heat to the inside of the block.
It is entirely consistent for the engine to appear to run well, rejecting a certain amount of heat to the ice interface, while that interface freezes and cools further due to a greater heat loss to the core.
The cold reservoir is in this case the core of the ice block and the distribution of its temperature is unknown.
Good explanation.
I more or less think that is probably what happened, but, in my mind, according to the Carnot theory, I figured the engine should melt the ice nearly as quickly as the ambient air itself. That 80% minimum pass through of "waste heat"
Also I had run the same engine in the same way, on the same ice in the same container type, everything the same possibly a dozen times already and nothing like that happened before.
The only thing different was the extra work the engine was doing, and possibly the timing might have been a little different.
As a reasonable alternative explanation though, I agree, maybe that's all it was.
1 minute ago, swansont said:So you’re coming up with a new definition of stuck? “For example, I say my engine got "stuck", apparently frozen.” But it wasn’t a mechanical part? How does a non-mechanical part get stuck? To be stuck, doesn’t it need to be something that normally moves?
The engine was on a double wall vacuum insulated cup full of ice (the cup was from Walmart a cheap makeshift Dewar)
The engine, started out at ambient temperature, about 80°F
I had the cup of ice in the freezer. Took it out of the freezer and set the engine on top. I was not in any particular hurry about this. By the time I started the engine the ice had already started melting and was slippery and the engine kept sliding off the wet ice.
I decided to use my usual experimental setup with the cup wrapped in insulation, styrofoam and blanket around the whole setup just to prevent the engine from sliding off the slippery wet ice.
Once I had the engine stable on the ice and able to run after adjusting the timing, I just let it run for a while.
Normally with that setup, I can very easily just lift the engine up off of the cup of ice.
This time when I went to check on the progress to see if the piston was more free to move or not with the honing job, when I went to lift the engine, I couldn't. It had become stuck to the ice. It was stuck to the ice. Could not be moved. The bottom of the engine was frozen solid and stuck to the ice.
Like if you touch cold metal with a wet finger and your finger gets stuck to the cold metal.
Cambridge dictionary for "stuck"
Quoteunable to move from a particular position or place
The engine could not be lifted from it's position on the ice, it was stuck to the ice.
This is why I think a video can be valuable, no lengthy explanation required, no confusion about the meaning of words.
0 -
3 hours ago, Ghideon said:
Recommendation: don't focus too much on ice based experiments unless you have some way to properly control the temperature of the ice. There seem to be many sources of errors which allows for different interpretations and varying outcomes.
If this were under government contract or grant or something maybe we could milk it for all it's worth I suppose, but with nearly zero budget at my disposal, ice will probably have to do for now.
BTW you might be interested to know that I just posted your idea to the Stirling Engine forum as THE solution.
https://stirlingengineforum.com/viewtopic.php?f=1&t=478&p=18490#p18490
Hope you have no objections, and hopefully not against forum rules.
I think it may be the longest running (still active) thread on the forum. (Since 2010).
42 minutes ago, swansont said:Mechanical devices getting stuck violate NO principles of known science.
The mechanical part did not get stuck. The engine continued running.
The engine running "on ice" (ambient heat) "stuck" to the ice which re-froze. Ice that had already begun to melt. It required considerable force to break the engine free.
I'd break the engine loose, watch the ice get wet and slippery, starting to melt, put the now WARM engine back on the ice and the ice would freeze again. The engine would eventually get frozen solid to the ice so it could not be lifted off. That took about ten minutes.
You can dismiss that with your flippant comment as if it's nothing, but I was there, I had to pry the engine loose from the ice over and over. There was no mechanical failure with the engine. It kept running the entire time.
0 -
1 hour ago, Ghideon said:
That seems reasonable. But under the insulation the temperature will be rather stable once the Stirling engine has run for a while? Temperature will likely vary depending on the load and added heat (and more) but if the effect you propose is there the temperature should read below ambient if the insulation is good enough. Initially it is good enough tho show experimentally, beyond doubt and in a repeatable fashion, that effect you predict exits; exact numbers can be found in later experiments?
Well, it is already widely accepted fact that a Stirling engine acts as a heat pump when turned over manually, in the same way and in the same direction that it normally runs as an engine.
There are many, many, many different heat engines or "hot air" engines out there that are lumped together as "Stirling". To name a few, the LTD (Stirling) engine is of rather recent origin as is the Ringbom (Stirling) the "thermoacoustic" (Stirling), Free Piston, free displacer, "Rice"-thermoacoustic (literally the rice people eat) is absolutely astonishing, Laminar flow, Thermal lag, "metronome", fluidine etc etc aside from the broad categories or designations alph, beta, gamma, so making broad generalizations about what "will" happen or what is "predicted" is misleading at best.
Most of the engines I purchased for experimenting with were immediately modified. Metal heat conducting bolts replaced with non-heat conducting nylon bolts. The timing/advance angle manually set to the "best" position in my own estimation based on the resulting performance and so forth.
In the case of the ice freezing (apparently) I don't know exactly how I set the timing.
My plan was to put some grinding compound on the piston/power cylinder and let it run on ice until it ran more freely to break in the new epoxy piston.
I had to adjust the timing several times to a position where the engine would run, the piston intentionally gummed up with grinding paste. I don't know exactly what I set the advance to as I did not intend that to be a controlled experiment, I was just attempting to break in the new epoxy piston in preparation for using it in a future experiment.
I'm quite sure the "conditions" are fairly easily reproducible (for someone who has some idea what they are doing), but these things take time and money. I just sent away for that upside down engine for $60 for example.
My point is, I don't actually know what caused the ice to re-freeze. (Four times in 45 minutes). But it sure didn't seem like a lot of heat was being pumped through the engine.
The ice came straight out of the freezer so was probably well below 0°C.
Possibly there was enough cold in the ice to cause it to refreeze when covered or re-covered over after beginning to melt on the surface. But like I say, the engine was room temperature. Just weird that the ice should keep re-freezing IMO if the engine is passing heat through to the ice AT ALL while running.
Personally, I think your idea of putting the cold sides of two engines back to back is brilliant and virtually fool proof.
If some ice is used to get the engines going, well, it either melts eventually or it doesn't. If it melts, that can't really be attributed to insulation leakage if the only thing on the other side of the engine is another identical engine.
Honestly I've been scratching my head over the problem for years already and you come up with a solution almost instantly.
It is, theoretically, like having two refrigerator/freezers back to back or front to front chilling the same freezer compartment. How could it not get cold if the freezers are actually functional. Better really. Conventional freezers still require insulation. This could be arranged in a way that doesn't.
You are a genius.
0 -
53 minutes ago, swansont said:
What thermodynamic principle does an engine getting stuck demonstrate?
Probably none. That's the point. It's an anomaly that appears to run contrary to "known science".
The engine is supposed to be, according to accepted scientific principles, dumping heat into the ice.
The engine was at room temperature. About 80°F at the time it was first placed on the ice. The ice was already begining to melt. Instead of melting the ice more with the heat, presumably being transfered, the ice re-froze, not just once but four times in the course of about 45 minutes observation.
Possibly it demonstrated cooling by expansion work would be my hypothesis.
24 minutes ago, Ghideon said:I think your (rather long) answers essentially answers my question with "yes, the cold side of the Stirling engine will cool below ambient temperature if sufficiently insulated."
Using a thermometer under the isolation on the cold side should be able to show this. Note that if the temperature probe is too large and is made of a material that transfers heat it may disturb the experiment.
Recommendation: don't focus too much on ice based experiments unless you have some way to properly control the temperature of the ice. There seem to be many sources of errors which allows for different interpretations and varying outcomes.
But you seem to claim that every single engineer* in the whole world the last 200 years are mistaken?
*) and scientist, teachers and others...
There are a lot of additional Ifs to consider. If the engine timing is adjusted properly, if there is proper load balancing (not a lot of excess heat) If the engine is performing sufficient work output, off the top of my head.
A Stirling engine will begin acting as a heat pump and begin creating a temperature differential just by turning it over with your finger to get it started to some degree with no external ∆T applied.
A Stirling engine IS a Stirling heat pump, as far as the mechanics and structure of the machine itself
QuoteBut you seem to claim that every single engineer* in the whole world the last 200 years are mistaken?
Apparently. With a few notable exceptions.
Mistakes can be perpetuated indefinitely if no one ever questions it. Especially if they "seem right" or correct or are simply the best working theory or tool available.
Most science in fact is just an approximation to eventually be proven wrong.
0 -
1 hour ago, Bufofrog said:
And yet you seem to mostly ignore the input you receive.
Only posts that are generally counter productive, merely insulting, ad-hominem, derogatory etc.
Sometimes I don't have time to respond immediately but the posts keep accumulating rapid fire and I have to pick and choose.
I'm taking the time to answer this one now, though basically devoid of any useful or constructive content
If you can cite an important post I overlooked or "ignored" I'd appreciate it.
I try to answer everybody when possible.
What I find distasteful is moderators who use the power of their moderator status to punish and censor people that merely happen to have a different POV on a subject. Who cannot tolerate or even entertain the idea they could ever be mistaken.
0 -
On 1/30/2023 at 1:03 PM, Ghideon said:
I get the impression that your idea* allows for two Stirling engines to be mounted cold plate to cold plate to increase the efficiency of both the engines?
*) If correct that is...
These engines can operate inverted.

So a Stirling engine ice sandwich with bought engines right out of the box is not out of the question.
I might just go ahead and send for one of these upside down engines.
I like this source/manufacturer as the displacer timing/advance on these particular style of engines is easily adjustable.
They are also put together with ordinary nuts and bolts making modifications easier, if necessary.
0 -
1 hour ago, studiot said:
Could it also be to ask questions of those who know more than they do ?
Is that possible ?
Naturally.
That is the main reason I like posting on Science forums. For the feedback from trained scientists and mathematicians etc. who hopefully are curious, understand scientific methodology and can be unbiased and objective.
I don't have a formal adult scientific training but my father did. I practically grew up in his home chemistry lab as he was training for a position as a science instructor.
Kind of like growing up with Bill Nigh the science guy as your dad, along with my older brothers always doing experiments.
My mother on the other hand was deeply spiritual/religious.
Unfortunately forums are not as popular as they once were. Not sure why.
On one forum, after posting on it for years, the guy running the forum finally confessed he was actually the only real person on the forum. The rest were all made up characters. His "sock puppets". Not an unusual tactic to make the forum appear more active and interesting than it really is to anyone that might happen to wander in.
0 -
1 hour ago, exchemist said:
References to being shut down or banned, men in black (in your previous thread) and so forth. In the latest post it is this passage:
" I already had other threads here that were abruptly closed, then reprimanded and banned for simply "bringing up" the same.
I guess I'm doing it again, just mentioning I have a YouTube channel, apparently.
So damned if I do and damned if I don't (present my own evidence or theories anywhere in this forum)."
Unlike normal people, who just get on with discussing the science, you whine about moderation policy, casting yourself in the role of victim. Give us all a break from that. I'm far more interested in your wrong-headed arguments, especially when they get me to explore bits of the history of science that are unfamiliar to me. 😀
Having a beef with draconian moderation policies applied arbitrarily and making mention of the fact is not "paranoia".
Uhhh.. "men in black" in a previous thread?
What was the context?
No doubt another joke or something you took too seriously.
You only NOW come to the realization that Carnot viewed heat as a fluid and a heat engine as some kind of water mill heat flowed through?
This was after I simply made casual MENTION of one of my YouTube videos that happened to be in an old closed thread.
Banned for simply making casual MENTION of one of my videos of an experiment.
What exactly is the purpose of someone starting a thread on a forum anywhere other than to make a presentation of some ideas.
I was continually bludgeoned with "moderator notes" and warnings for simply doing what people generally do on forums. Have a discussion or try to.
-1 -
I said the moderation policies around here "cramp my style". I like video. I have dozens of recorded video experiments. It answers toward credibility so others can see what I'm talking about and judge for themselves.
For example, I say my engine got "stuck", apparently frozen.
People can watch the video and judge for themselves, they don't have to take my word for it.
I've been banned from the forum previously for simply mentioning or "bringing up" a video. That is not paranoia that's FACT.
-1 -
As I saw it, what was needed is some form of metering device.
If ice were used as the "sink" or cold reservoir, the flow of heat into the "reservoir" could be determined on the basis of the rate of ice melt.
The running vs. not running engine type test could at least demonstrate some difference.
A very low budget solution.
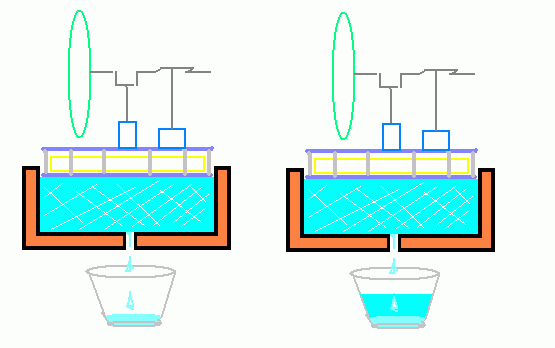
The result of this experiment has already been described above. The ice took longer to melt with a running engine on top vs. an idle one.
In the first trial of this type, the ice took, if I recall, 27 hours to completely melt under the inoperative engine and 33 hours to melt under the running engine.
This only confirms known principles however and is unremarkable IMO.

*
With a running engine converting SOME of the heat to work output, less heat is "rejected" to the sink. (The ice melts more slowly).
I surmised however that perhaps, since heat is NOT a fluid and zero heat is literally "flowing" into the sink, there is a good chance that the majority of the ice melt in either instance is primarily due to imperfect insulation.
That is, perhaps the ice under the running engine is .melting more due to heat infiltration through the insulation rather than heat "flow" through the engine.
I've been puzzling on how to overcome this imperfect insulation issue.
The adjoined engine idea suggested previously affords a real solution to this problem.
Another engine might act as a "perfect" insulator. The heat to melt the ice would have to pass through one engine or the other.
A minimum of four identical engines would be required to be able to run (and not run) them concurrently.
We could get away with two if the experiments were run sequentially.
Engines of special design might however be necessary.
Setting up such an experiment could take some time.
*The above heat flow image BTE was originally from this site: http://labman.phys.utk.edu/phys221core/modules/m10/engines.html
The link is simply provided for credits, though probably a stock image anyway. There are no copyright notices displayed on the page, but I do like to cite my sources.
2 hours ago, exchemist said:Hmm. One of the more annoying features of your posting style is the way your evident paranoia occasionally breaks through.
Paranoia ?
And exactly what in my post do you consider "evident" of paranoia ?
0 -
I made an effort at running an engine using this arrangement:
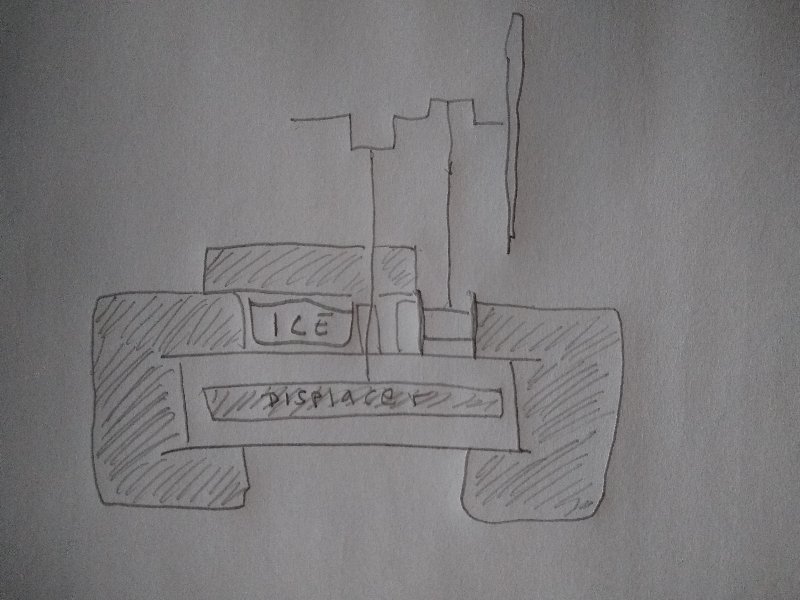
The engine is operating with a small pocket on top to hold an ice cube.
Below, is an opening to admit ambient heat.
I thought the advantage of this arrangement is that the engine could run in the same direction it was designed to run in, with the heat source on the bottom plate.
Other than the opening on bottom for heat input the engine was otherwise encased in insulation.
The ice cube would act to initiate a ∆T but once in operation, would the ice melt?
If the top of the engine was being refrigerated by the engine itself, the ice should take some extra time to melt at least.
Well the engine started and ran under it's dome of insulation and seemed to be churning away to beat the band, running quite well.
I carried the engine through the house and up the stairs to show my wife the successful new arrangement, rather jubilant I might add.
Then as I was animatedly explaining what was going on in the middle of the conversation the engine abruptly seized.
I was like "what the heck..." Perhaps in not such mild language
My wife interjected with the comment:
"Maybe it froze up again"
I can't actually confirm that, but I was not able to get the engine restarted.
I took it back down stairs to examine. (The "lid" was secured down with tape)
By the time I got the lid off the ice appeared to be rapidly melting, but still some ice in there.
I cleaned up the engine and it was able to run normally again.
I was not able to draw any conclusion from these results.
The experimental arrangement however was tentatively a success if the apparent "freezing up" of the power cylinder could somehow be avoided. A materials issue, probably.
Did it literally freeze up, due to excessive cooling? That was hard to believe, even for me.
Convinced however, that there is a "resistance" to heat flow or "pushing back" of heat by the engine and that the engine is certainly converting rather than transmitting heat primarily, I have set about designing a new flavor of heat engine NOT based on the Carnot concept of heat flowing through the engine like water.
A hot side only arrangement with no cold side.
Here is one arrangement I'm currently in the process of building.
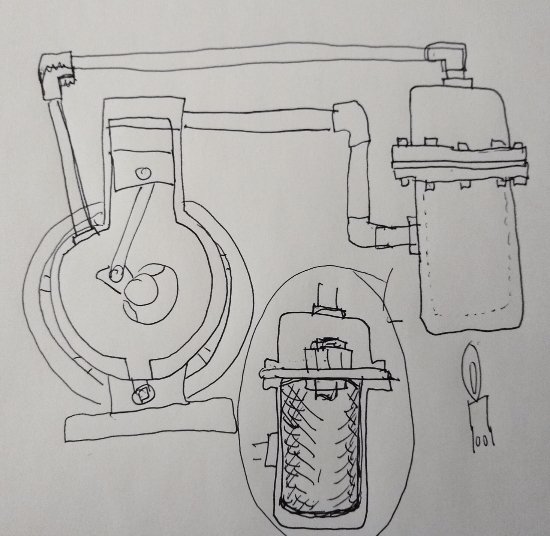
The project is a fairly simple conversion of an air compressor into this new "sink"-less external combustion engine.
Rather than a COLD side, the cold sink for "waste heat" has been replaced by a rather simple air spring. lacking any functional outlet for "waste heat' as no waste heat is anticipated.
Secondary heat of compression in the air spring should simply be returned to the engine.
Time will tell if this idea actually works or not.
I'm also working simultaneously on a twin compressor conversion.

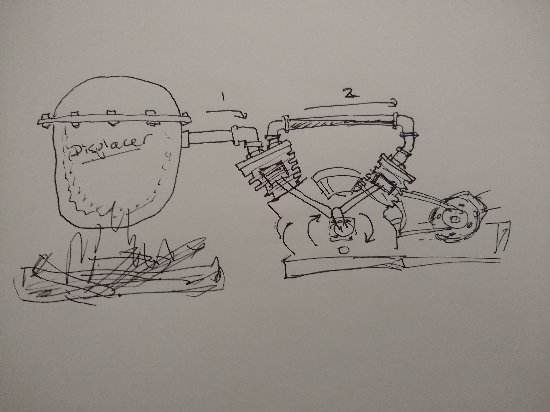
The valved cylinder heads will be replaced using simple steel plates

Enough about my theories and conversion projects.
How can we demonstrate the percentage of waste heat predicted by the Carnot efficiency equation is accurate?
0 -
Speaking in broad terms, in designing a Stirling engine, It would seemingly be important to know how the engine actually functions.
Apparently, not like a water wheel so much as a dam that rather than facilitating heat flow, causes it to back up. Resists the natural direction of heat migration, with the exception of one outlet. The piston.
The difference though, between a Stirling engine and a turbine in a penstock is that there is not actually any material fluid flow through the piston Only transfer of kinetic energy.
My little engine in this experiment came with a graphite piston. Graphite is an excellent conductor of heat, better than steel, comparable to aluminium. Probably not the best choice if the idea is to contain heat but convey kinetic energy to a crankshaft.
If a design goal is to resist heat flow through the engine, the last thing anyone should want to intentionally incorporate would be a heat sink or cooling jacket, That would be like blowing a big hole in a hydroelectric dam providing an alternative path around the turbine.
I tried making a piston out of a non-conductive material once. Epoxy. Awful heat conductivity.
I ran the engine on ice with the epoxy piston, insulated, as usual.
The ice block had already started to melt and was slick on the surface.
After setting the engine on the ice and letting it run for about 15 minutes or so, I decided to pick up the engine to see what was happening and it was stuck.
The melted surface of the ice in contact with the engine had frozen. I had to forcibly break the engine loose.
Puzzled, I set it back and let it run another 15 minutes. It stuck to the ice again.
15 minutes later I went and got my phone and took a video, just in case it froze like that again.
It did, and I got my effort to break it loose on camera.
It happened again after the video. Every time after breaking the engine loose, the ice became slick from the heat in the room, but then with the engine back running on top, the surface of the ice in contact with the engine re-froze.
I had previously run the same engines on ice numerous times, but never had anything like that happen before.
At the time that epoxy piston was formed by pouring liquid epoxy into the cylinder and letting it cure, so the piston was extremely tight.
I had also been running the engine, not as an experiment, but just to break in the piston.
I had smeared the piston and cylinder with grinding compound. (A thick paste with carbide grit) so that the piston and cylinder would have a better fit.
After taking the video and having the ice refreeze one more time I let the engine run the rest of the night and went to bed.
By morning the engine had stopped and the ice was melted but the piston and cylinder were ruined.
0 -
As far as "atmospheric pressure", it has crossed my mind that a perfectly horizontal line is probably slightly inaccurate, by how much I couldn't say.
In other words the piston moves out against atmospheric pressure. How much of the atmosphere is actually displaced and to what extent?
Presumably this would cause a localized pressure increase in the cylinder immediately above (on the outside/atmosphere side of) the piston.
During contraction of the working fluid the returning atmosphere rushing into the cylinder wod likely be trailing behind somewhat, so effectively, "atmospheric pressure" acting on the piston would not actually be entirely stable but would increase slightly with expansion of the working fluid and decrease slightly with contraction.
Significance? Probably none. Possibly a slight heating and cooling of the air above the piston in reverse of the heating and cooling of the internal gas.
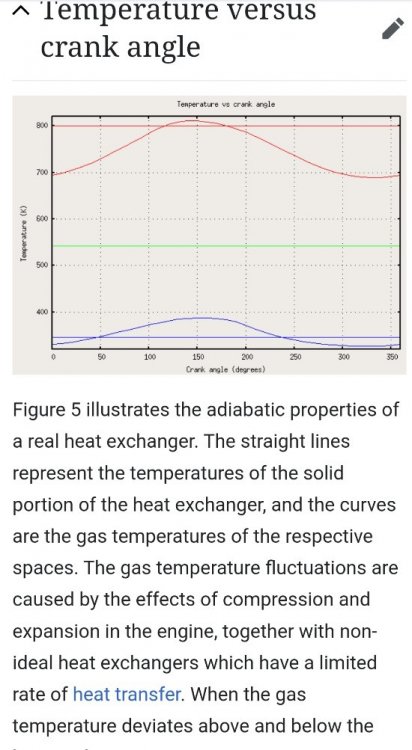
Well, my infrared camera showed a localized "hot spot" in the power piston area. Friction? Localized compression? Maybe the Hot interior of the engine showing where insulation was lacking at the piston?
The isotherms superimposed are only meant to be vaguely suggestive.
Likely the temperature is not uniform. Remember the working fluid is divided roughly into two compartments one hot and the other cold
In my chart, the snowflake/crystals are meant to represent that the cold side working fluid has dropped in temperature below the temperature of the heat exchanger. At that point however the working fluid has been displaced almost entirely to the cold side.
Logically the engine takes in or absorbs some heat into the cold working fluid at that point. But the heat source is covered.
Conclusion?
The cold side "sink" is being chilled during expansion similarly to how an expanding refrigerant chills a refrigerator.
Likewise we see the reverse during compression. Some heat is being returned to the hot side back to the heat source.
The engine is acting as a heat pump.
Google, in a real word attempted application, wanted to use Stirling engines on their data centers to cool the building and generate electricity.
It didn't work out.
The Stirling engines acted like insulators, keeping the data center hot.
So what does this do to "...a fixed amount of gas doing work by expanding against a piston and then being cooled so that it can repeat the cycle and do more work."
"Being cooled" ?
Is the gas "being cooled" by the sink?
How is that possible if the working fluid is actually refrigerating the sink, acting like a heat pump?
I'm not trying to prove a theory, just drawing tentative conclusions based on the available data.
0 -
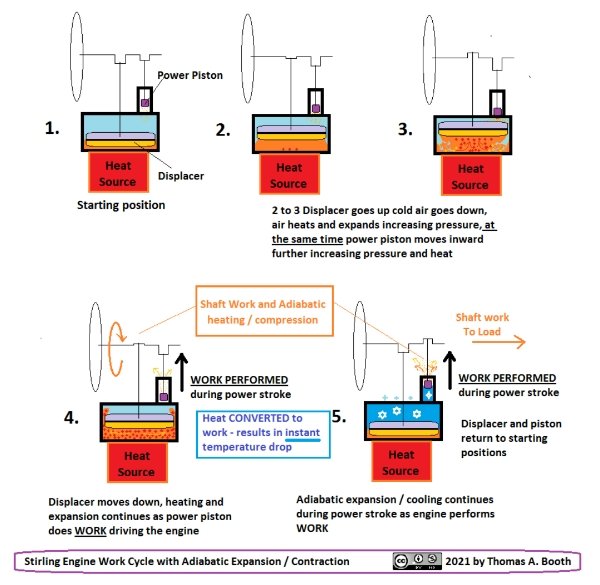
Maybe it would be OK to post these two images:
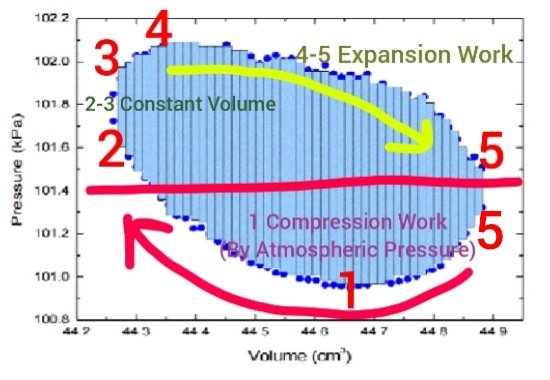
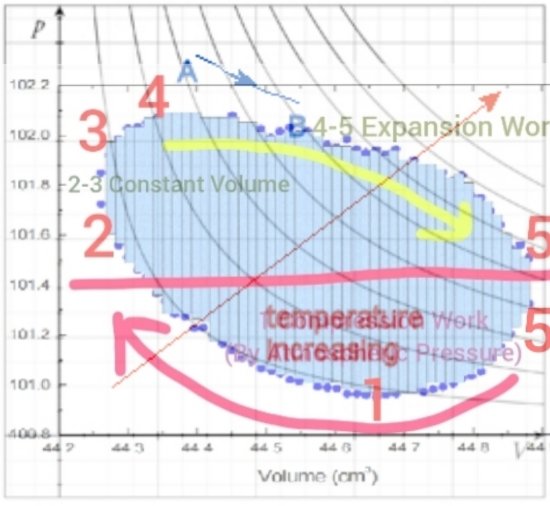
The first image is an actual real-time PV diagram generated by sensors placed on or in a running Stirling engine (again LTD type as in my experiment presented here) the isotherms however are from a different chart superimposed over the actual PV diagrams so should not be considered in any way real or accurate but just my attempt to get some idea what might be going on temperature wise I side the engine.
The second diagram is my attempt at portraying my "alternative" Stirling engine cycle.
That was done a fairly long time ago however and I should probably add a few details and explanatory notes, but more or less still representative of what I THINK probably goes on inside (and to some degree outside) a running Stirling engine.
The numbers appended to both 1,2,3,4,5 relate one chart to the other.
#5 is duplicated on the PV diagram because it represents that entire section.
In other words that entire section 5,5 in the PV diagram is what is described and pictured in the other chart at #5 where the cycle cuts across the isotherms and a rather steep cooling occurs due to expansion work.
I called this adiabatic but that is only because that was the best description I had at the time but there naturally would be some heat transfer by both work on the outside atmosphere by the piston as well as simultaneously into the engine due to the colder than ambient temperature reached by the working fluid. Some relatively minor details are not specifically represented.
The words "Temperature Increasing" are from the superimposed isotherm chart and is not part of my evaluation but refers to the diagonal red arrow showing the direction of temperature increase for the isotherms.
The red horizontal line, my estimate of where atmospheric pressure might be, should probably be a bit lower. The actual weather conditions, atmospheric pressure at the time readings were taken are unknown (to me at this time).
If a clear day with a high pressure system in the area, perhaps it is close to being accurate, at any rate the diagram and the atmospheric pressure is typical of other similar real-time PV recordings of Stirling engines (one posted earlier for example where atmospheric pressure was represented by the horizontal line at 0.
0 -
Carnot efficiency properly applies to the Carnot engine, I suppose, but allegedly the Carnot engine has an efficiency that NO OTHER engine can exceed.
Stirling engines are at least, theoretically supposed to be able to approach the carnot limit, but at any rate a Stirling engine being probably less efficient, but certainly no more efficient, should if anything "reject" MORE heat at the sink not less than predicted by the equation, so therefore should be suitable as a stand in for a Carnot engine.
Would it be possible for a Stirling engine to be LESS efficient than a Carnot engine and yet also "reject" less heat, percentage wise?
I'll concede, we don't have enough information to draw a conclusion at this point. So how to proceed?
I think the idea of putting two (or more) engines with the sink sides adjoined would largely solve the problem of ambient heat infiltration from the environment. No heat would be able to reach the 'sink" except through either one or the other engine. This simplifies measurements by eliminating the ambient "wildcard" contributing unknown quantities of heat into the "cold reservoir".
Other suggestions for comparative analysis, measuring heat transfers with the engine running vs. not running might yield additional data.
I attempted such a comparative experiment previously which I ran several times using different quantities of ice.
I placed a non-operating Stirling engine on a cup of ice (surrounded by insulation) and recorded the time it took for the ice to melt. Then repeated the experiment with the engine running.
In every instance, running/not-running the engines on cups of ice, as well as single ice cubes added to cups of ice water, the ice or ice cube invariably took longer to melt when under a running engine vs. the "dummy" inoperative engine.
Most people on the forum predicted the opposite result, due to the running engine actively churning the working fluid, presumably transporting heat to the sink in an active way, while the "dummy" engine was just sitting there.
A review of all the tests indicated that the ice took 15% more time to melt under the running engine than under the inoperative engine.
43 minutes ago, Ghideon said:I did not ask for a complete explanation or theory, just if you had an idea what to look for in the experiment or what to expect; it may trigger new ideas about how and where to measure. (...)
I've developed a more or less complete theory of an alternative "Stirling" engine cycle, to be sure. But I'm not here to promote that.
I was previously banned from the forum for "soapboxing" for, I assume, that very reason.
Since 100% of my "evidence" is digital video recordings and other data I've made widely available on the internet, I'm not allowed to post or link to here it puts something of a cramp in my style.
However in attempting to obey the forum fules, I'm then accused of being "reluctant" to present my theories and evidence or "running scared"
At any rate MY theories are NOT the Carnot limit equation, were developed entirely independently from my own observations, research and experiments, some of which includes making various modifications to "stock" model engines.
I'm not at all "reluctant", rather I feel severely hampered by the restrictions. No video, no links? Presenting experimental video recorded evidence is soapboxing?
My explanations must conform to previously known "ideal" processes (isothermal or adiabatic) well sorry but if those are the rules, discussion of any novel findings is rendered impossible. That is not due to me running away. But it's not really appropriate to a thread devoted to demonstrating the factuality of the Carnot equation either.
Best I feel I could do is start another thread and C/P the data I've already posted or uploaded from other forums, and maybe post written descriptions of the experiments, but I already had other threads here that were abruptly closed, then reprimanded and banned for simply "bringing up" the same.
I guess I'm doing it again, just mentioning I have a YouTube channel, apparently.
So damned if I do and damned if I don't (present my own evidence or theories anywhere in this forum).
0 -
20 minutes ago, Ghideon said:
Thanks for the reply. some further reasoning about the experiment: Let's again assume one engine, per your initial setup. Insulation is good and we have means to measure the temperature at the cold side and the hot side. At the start the cold side temperature is same as ambient (room) temperature.
What should we look for when the engine is started? Is it correct that you predict that the temperature will drop below ambient when engine is running*?
Assume the heat added on the hot side is not the maximum what the engine can handle. So therefore we can increase the temperature on hot side . What result do you predict on the cold side? Will the temperature drop further on the cold, isolated side? I ask since this could be easier to measure; we may check the temperature differences and trends and maybe easier get rid of errors.
(* I am aware of the implications of this ...)
That there might actually be a refrigerating effect at the cold heat exchanger, I would prefer to consider unproven, untested wild speculation that goes way beyond the simple demonstration of the deposition of waste heat predicted by the Carnot (so-called) efficiency (so-called) equation.
A point brought up is that we don't actually know the exact amount of heat going into the engine. We don't know the actual work being done, so it is not possible to make assumptions about any temperature readings at the sink.
To my mind 80% of the heat supplied from actively hot boiling water, (not just a warm cup of coffee on its way to cooling down) is way over the limit of what this little engine is rated to handle by the manufacturer. (I think the specifications state no more than 80°C if I recall correctly, I can check on that)
But, to be fair, the working fluid is air, which is an extremely poor conductor.
What differentiates things though is inside the engine the displacer is being worked up and down continually accelerating convective heat transfer into the engine (presumably). The sink has been insulated with progressively higher and higher grades of insulation. (presumably)
It is not inconceivable that acting as an absorber/conductor Aerogel, or other insulating material might actually serve to somewhat INCREASE heat conduction away from the engine infact also increasing surface area for heat dissipation to the actual or final ambient "sink".
I don't contest the validity of such arguments (much).
All I know is that the result MANY Stirling engine enthusiasts and model engine builders on the forum expected, (including myself to a great degree) did not take place as predicted.
Instead, in earlier experiments, the engine actually ran faster, as my previous outlandish theory about expansion work cooling predicted.
I'm honestly not trying to "prove" any one theory over another, I would just like to know, is this Carnot efficiency something that can be demonstrated experimentally.
I thought my insulation idea was a surefire test, but apparently not so much.
I suppose proving an alternative hypothesis would tend to negate the former view, but to me that seems like a long shot, and proving me wrong does not exactly validate the Carnot efficiency formula.
After 200 years, demonstrating the correctness of the formula experimentally should not be difficult. It should perhaps be a routine high school science class experiment.
Perhaps it is, but if so, why withhold that information?
0



Is Carnot efficiency valid?
in Speculations
Posted
Great, and I'm more than happy to prove my hypothesis wrong. So IMO it's a fantastic suggestion for an actual experiment.
There is though, I believe, at least one very real example of a "self-running" cooler/heat engine utilizing evaporative cooling.
Could a similar device utilizing a compression expansion cycle for refrigeration instead of evaporative cooling be devised or actually exist?
I'm not entirely convinced that such a thing is impossible, especially since finding out that a rather intelligent guy who pretty much handed us the modern world on a silver platter thought it could work.
I'm not making any claims whatsoever. I just have a hunch that maybe he was just a little smarter than some of those other guys; Carnot, Kelvin et al
Not that your addressing me I guess but it seems to me that a small bit of ice sandwiched between the cold sides of a couple of Stirling engines is a no-brainer.
Either the ice melts or it doesn't.
I'm willing to concede that if the ice melts that proves my theory wrong conclusively. The engine is not a heat pump pulling heat from the cold side. We can all go home.