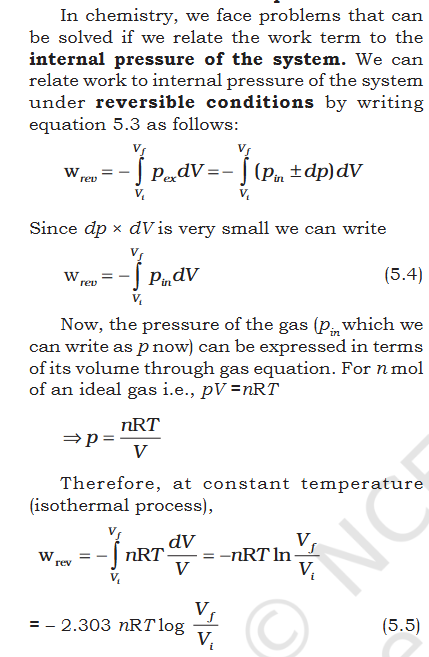Everything posted by HbWhi5F
-
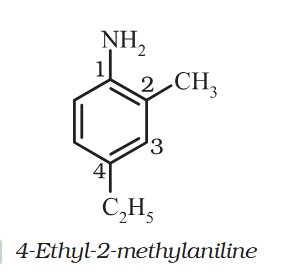
4-Ethyl-2-methylaniline: Why is C2H5 called Ethyl ? and Alternative names ?
Ethyl is C2H6. Why why C2H5 is called that here ? Aniline means benzenamine (C₆H₅NH₂) but NH2 is at 1. So it can also be written as 1-amino-4-Ethyl-2-methybenzene ?
-
Why assume hybridization and not charges on Carbon ?
Example if carbon is bonds to 3 species we assume it's singly bonded with 2 and doubly bonded with 1 and has hybridization of sp2. Why don't we assume sp3 and there is a negative charge on C ? Is it role of hybridization is to form maximum sigma bonds ? All non-hybridized orbitals form pie bonds ?
-
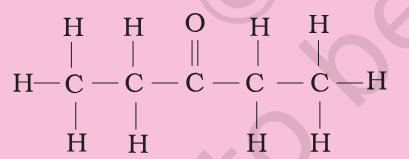
How to find no. of bonds between C and O ?
@chenbeier When are triple bonds formed by O ?
-
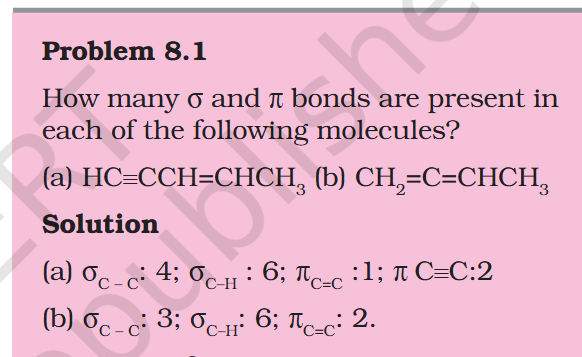
Is Pie bond of Double and Triple Bond CC different ?
@studiot To reiterate the question, the one pie bond in a double bond and 2 pie bonds in a triple bond are they different. I get the hybridization.
-
How to find no. of bonds between C and O ?
-
Is Pie bond of Double and Triple Bond CC different ?
In (a) Is the Pie bond of Double and Triple Bond CC different that is why written separately in answer
-
Need help with derivation of Solubility Product Constant of weak acid. S = {Ksp (H + Ka) / KA}^1/2
Firstly there are what seems to be typos, H has negative charge the latter inverse equation are X instead of H in denominator. Question solubility is for individual ions for salt they is solubility product Ksp ? What is S [f S] and why that equalts to Ksp and S^2 {KS/(Ks + H)} and how is that equal to S = {Ksp (H + Ka) / KA}^1/2 I took me 2+ hours to try and understand this but still don't get it.
-
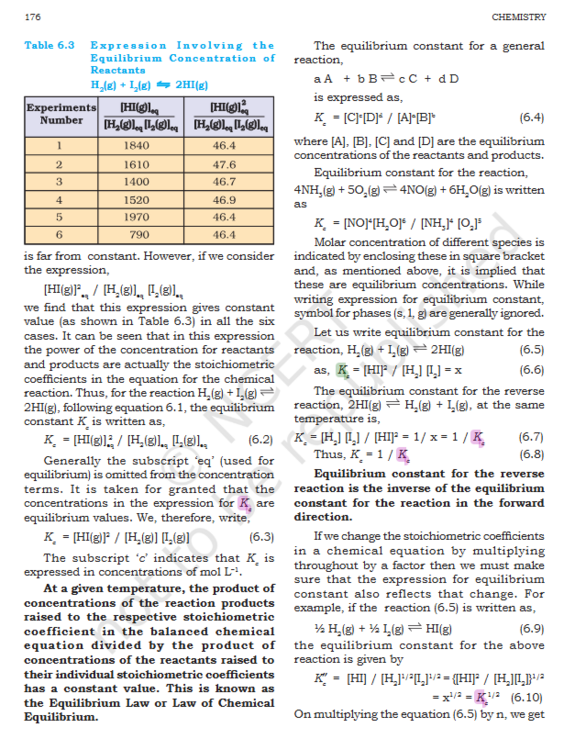
Equilibrium: Why is products assumed to formed in equal amounts ?
@KJW thanks
-
Equilibrium: When Pressure is increased x2 is the every species multiplied by 2 ?
- Equilibrium: Is Kc=1/Kc always true ?
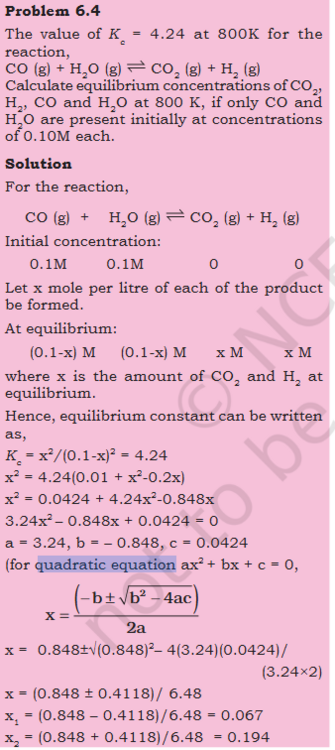
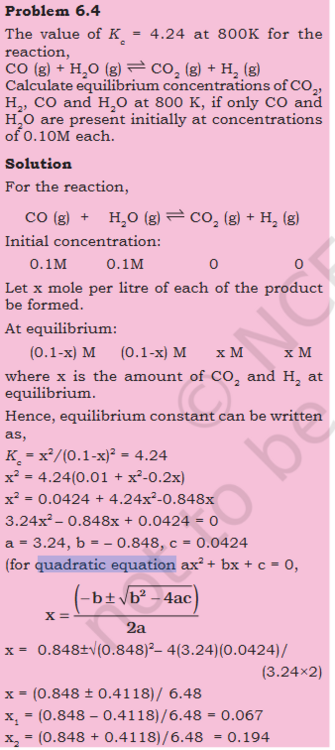
- Possible multiple both side by -1 to change sign when RHS has 0 ? a−b+c=-a+b-c
Original question Can one multiply both sides by -1 to flip signs ? Steps - Here RHS is subtracted from both sides, giving us 0 on 1 side x^2- 4.24^2 is combined both sides are multiplied by -1, to flip the signs. that seems like cheating, just flipping signs. If LHS where subtracted from the both sides we would have still got the same result ?- Equilibrium: Is Kc=1/Kc always true ?
- Equilibrium: Why is products assumed to formed in equal amounts ?
- Equilibrium: Is Kc=1/Kc always true ?
If yes what does this mean ? What would the graph look like ?- Possible multiple both side by -1 to change sign when RHS has 0 ? a−b+c=-a+b-c
What makes me think this - Here RHS is substracted from both sides and x^2 terms are also combined. then both sides are multiplied by -1 that seems like cheating, just flipping signs.- How to prepare pdf for AI (Notebook/Flashcard). Coping text from PDF with scientific symbols not working.
Problem"explain entropy as a" is copied as "e x p l a i n e n t r o p y a s a". sometimes it "seems" like homoglyph-like character. example - letter "a" and the Cyrillic letter "а" there are random line breaks everywhere. Scientific symbols are not copied or copied as . specially super/sub-scripts. Sigma Symbol is not copied at all. Sometimes selecting is hard selecting formula selects everything or otherthings Superscript +/- are not copied. Arrow is not copied always, seems like sometype of DRM the book it using 2 different looking arrows. There is sometimes what seems to be hand written Symbols I copied "minus in a circle in superscript" to https://www.soscisurvey.de/tools/view-chars.php and it shows as U+F030, which https://www.compart.com/en/unicode/U+F030 as it for private use Notes/QuestionI have 2 problem -Not able to copy correctly Prepare pdf for LLM SituationText is copiable not using OCR. The text is already copiable but I want to add OCR layer to it how ? Make OCR ignore footer/header and page number Example Pdf - The pdf is free to use for personal use but illegal to print. https://ncert.nic.in/textbook.php?kech1=5-6 What I Foundhttps://github.com/datalab-to/marker 3. What is the best way to convert the pdf to flashcardI tired https://anki-decks.com/app/dashboard/ but it limited to 25pages and doesn't ask the important things (doesn't understand the context for science to get formulas and tables) symbols are not working - Pdf weirdness image Does this has to do it Unicode and pdf software ?- is x-y = |y-x| ?
@MigL What is the law called ? Seems useful but never taught- Why is work done negative and more then it's in finite steps ?
@exchemist why is it more when done in finite steps ? The same process takes more energy ?- Enthalpy of Dilution: Hess's Law, Subtracting 2 Equations, Negative Value of endothermic.
@exchemist why is it negative and book says process is endothermic ?- Entropy: "Heat added to system at lower temp causes greater randomness than when same quantity of heat is added at higher temp" ?
@swansont This is from NCERT Class 11 Chapter 5 @MigL Studying- Entropy: "Heat added to system at lower temp causes greater randomness than when same quantity of heat is added at higher temp" ?
"Heat added to a system at lower temperature causes greater randomness than when the same quantity of heat is added to it at higher temperature. " this can mean 2 things heating a colder object causes more randomness or supplying same amount of heat slowly causes more randomness I think the 2nd one because ∆S = qrev/T- Enthalpy of Dilution: Hess's Law, Subtracting 2 Equations, Negative Value of endothermic.
Book says - "(f) Enthalpy of Dilution It is known that enthalpy of solution is the enthalpy change associated with the addition of a specified amount of solute to the specified amount of solvent at a constant temperature and pressure. This argument can be applied to any solvent with slight modification. Enthalpy change for dissolving one mole of gaseous hydrogen chloride in 10 mol of water can be represented by the following equation. For convenience we will use the symbol aq. for water HCl(g) + 10 aq. → HCl.10 aq. ∆H = –69.01 kJ / mol Let us consider the following set of enthalpy changes: (S-1) HCl(g) + 25 aq. → HCl.25 aq. ∆H = –72.03 kJ / mol (S-2) HCl(g) + 40 aq. → HCl.40 aq. ∆H = –72.79 kJ / mol (S-3) HCl(g) + ∞ aq. → HCl. ∞ aq. ∆H = –74.85 kJ / mol The values of ∆H show general dependence of the enthalpy of solution on amount of solvent. As more and more solvent is used, the enthalpy of solution approaches a limiting value, i.e, the value in infinitely dilute solution. For hydrochloric acid this value of ∆H is given above in equation (S-3). If we subtract the first equation (equation S-1) from the second equation (equation S-2) in the above set of equations, we obtain– HCl.25 aq. + 15 aq. → HCl.40 aq. ∆H = [ –72.79 – (–72.03)] kJ / mol = – 0.76 kJ / mol This value (–0.76kJ/mol) of ∆H is enthalpy of dilution. It is the heat withdrawn from the surroundings when additional solvent is added to the solution. The enthalpy of dilution of a solution is dependent on the original concentration of the solution and the amount of solvent added" Question What is the convention here I get the idea of dilution. Why it says to substract then goes on to add one side and subtract the other ? "It is the heat withdrawn from the surroundings when additional solvent is added to the solution." - the value is negative that means heat is given out ?- Why is work done negative and more then it's in finite steps ?
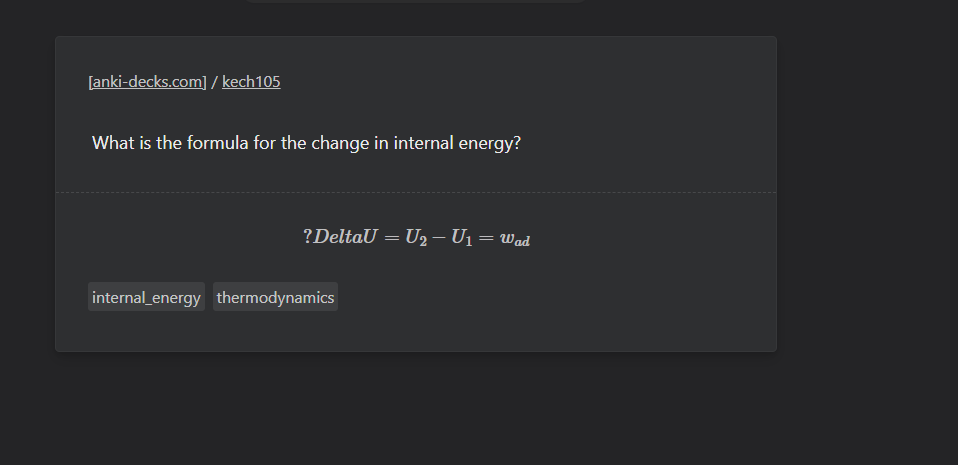
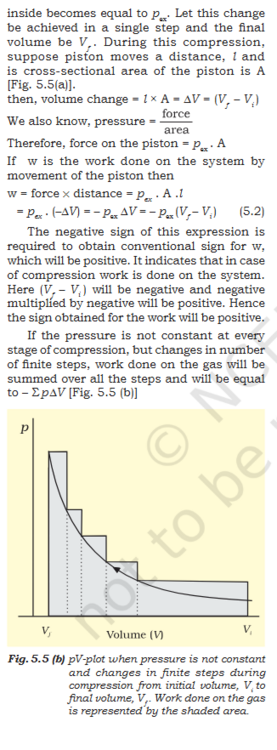
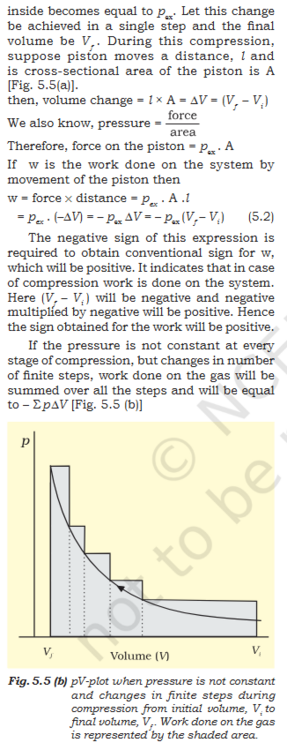
Book- "5.2.1 Work First of all, let us concentrate on the nature of work a system can do. We will consider only mechanical work i.e., pressure-volume work. For understanding pressure-volume work, let us consider a cylinder which contains one mole of an ideal gas fitted with a frictionless piston. Total volume of the gas is Vi and pressure of the gas inside is p. If external pressure is pex which is greater than p, piston is moved inward till the pressure inside becomes equal to ex. Let this change be achieved in a single step and the final volume be V f . During this compression, suppose piston moves a distance, l and is cross-sectional area of the piston is A [Fig. 5.5(a)]. then, volume change = l × A = ∆V = (V f – Vi ) We also know, pressure = Therefore, force on the piston = pex . A If w is the work done on the system by movement of the piston then w = force × distance = pex . A .l = p ex . (–∆V) = – pex ∆V = – pex (Vf – V i ) (5.2) The negative sign of this expression is required to obtain conventional sign for w, which will be positive. It indicates that in case of compression work is done on the system. Here (V f – V i ) will be negative and negative multiplied by negative will be positive. Hence the sign obtained for the work will be positive. If the pressure is not constant at every stage of compression, but changes in number of finite steps, work done on the gas will be summed over all the steps and will be equal to – Σ р ∆V [Fig. 5.5 (b)]"- Molecular Orbital: Need help conceptualizing.
@studiot Yes I would like to know about molecular bonds I didn't understand molecular bonds also I think c2 should form 1 sigma 1 sigma antibond 1 pie bonds and no antibonds but according to the mainthread (book) it froms 2 pie bonds .- Molecular Orbital: Need help conceptualizing.
@Stuart electron-electron repulsion ? - Equilibrium: Is Kc=1/Kc always true ?
Important Information
We have placed cookies on your device to help make this website better. You can adjust your cookie settings, otherwise we'll assume you're okay to continue.
Account
Navigation
Search
Configure browser push notifications
Chrome (Android)
- Tap the lock icon next to the address bar.
- Tap Permissions → Notifications.
- Adjust your preference.
Chrome (Desktop)
- Click the padlock icon in the address bar.
- Select Site settings.
- Find Notifications and adjust your preference.
Safari (iOS 16.4+)
- Ensure the site is installed via Add to Home Screen.
- Open Settings App → Notifications.
- Find your app name and adjust your preference.
Safari (macOS)
- Go to Safari → Preferences.
- Click the Websites tab.
- Select Notifications in the sidebar.
- Find this website and adjust your preference.
Edge (Android)
- Tap the lock icon next to the address bar.
- Tap Permissions.
- Find Notifications and adjust your preference.
Edge (Desktop)
- Click the padlock icon in the address bar.
- Click Permissions for this site.
- Find Notifications and adjust your preference.
Firefox (Android)
- Go to Settings → Site permissions.
- Tap Notifications.
- Find this site in the list and adjust your preference.
Firefox (Desktop)
- Open Firefox Settings.
- Search for Notifications.
- Find this site in the list and adjust your preference.