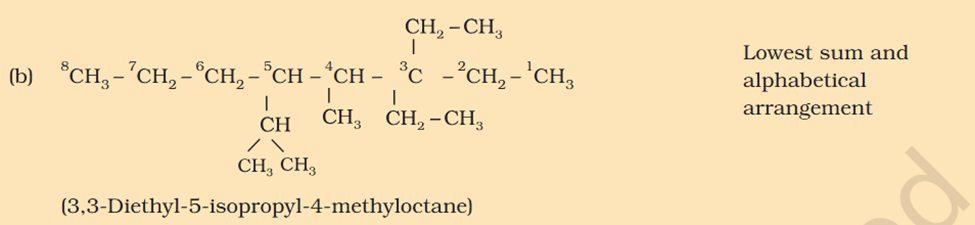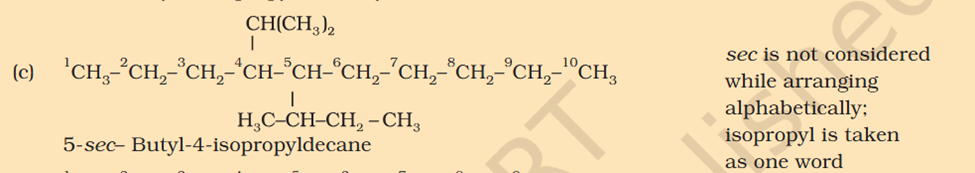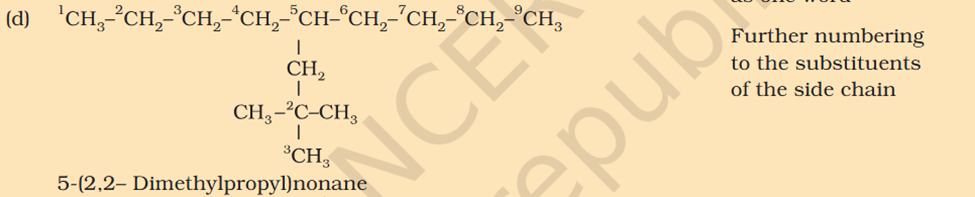Everything posted by HbWhi5F
-
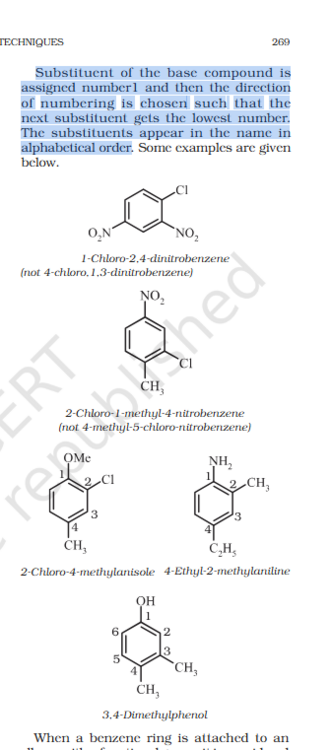
What is the Benzene base compound priority list ?
How to choose the base compound ? And start counting from that ? IS there is a prioty list ?
-
Alkenes general formula ?
i think this should be the answer but everywhere i looked it doesn't have +2
-
[Organic Chemistry] Nomenclature
Proply is c3h8 not 6 but in 2nd and 3rd example in main thread, in 3rd it means - propyl mean C3H5 Iso means 1 branch of methyl on 2nd but in 1st and 2nd example it doesn't follow that @exchemist
-
Benzene with polysubstituent nomenclature.
Is the book self-contradictory or am I tripping. Also what is the order of preference for base substituent ?
-
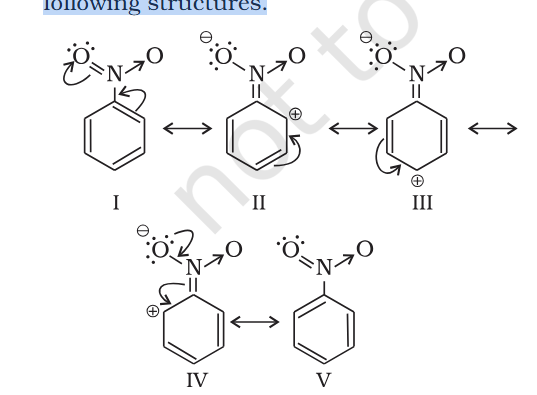
What is arrow from N to O in Nitrobenze Resonance Structure
@exchemist thanks alot you are my guru
-
What is arrow from N to O in Nitrobenze Resonance Structure
-
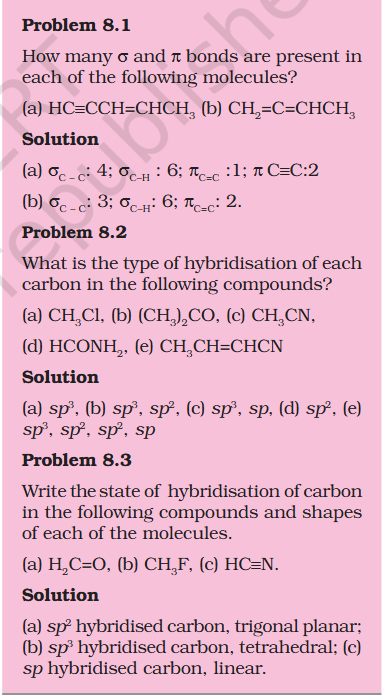
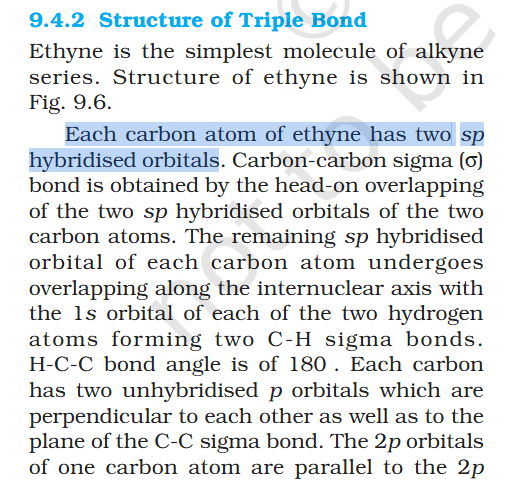
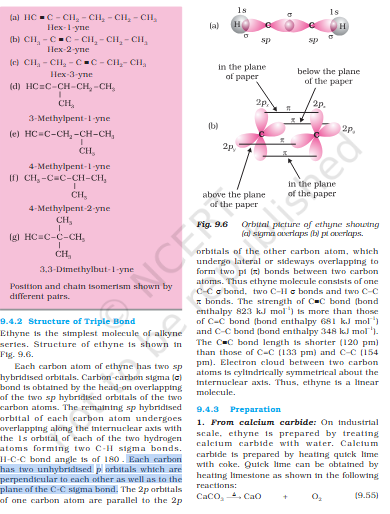
Pie bond uses 2 p orbitals ?
what does having x2 sp hybridized orbital mean ? In C-C triple bond x2 sp hybridized orbitals are formed, but in questions earlier it has taught that 1 sigma bond = s, 2 = sp, 3 = sp2 Uploading Attachment... Uploading Attachment...
-
Pie bond uses 2 p orbitals ?
-
Pie bond uses 2 p orbitals ?
@exchemist thanks
-
[Organic Chemistry] Nomenclature
@exchemist in 2nd example Propy is c3h8 not H6
-
Alkenes general formula ?
this makes me question alkane if CnH2n+2, so with 1 double bond should be (CnH2n+2)-2 removing 2 H (from adjacent C atoms) frees one 1 bond for double bond. If there are 2 double bonds then 2x2 H are lesser so (CnH2n+2)-2x2 For 3 double bonds (CnH2n+2)-2x3 Am i right @exchemist One day i wish i be able to pay you back for all the Knowledge and encouragement.
-
[Organic Chemistry] Nomenclature
@exchemist need help understanding nomenclature
-
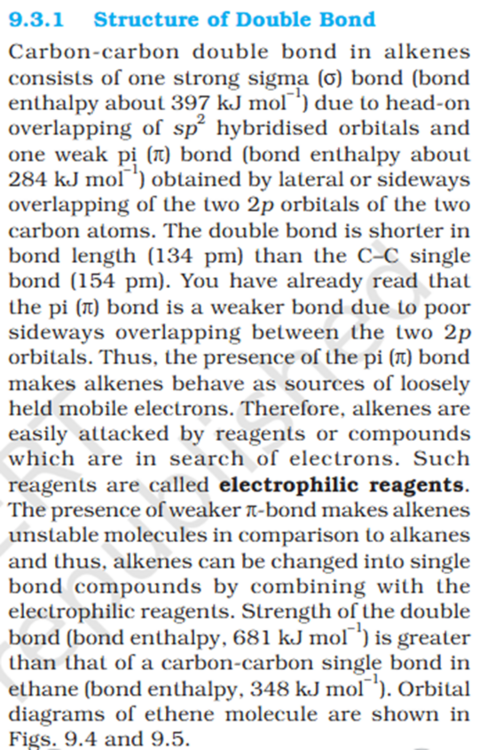
Why Carbon single bond is sp2 hybridized orbitals ?
Title
-
Pie bond uses 2 p orbitals ?
'You have already read that the pi (π) bond is a weaker bond due to poor sideways overlapping between the two 2p orbitals." 1 pie bond is actually 2 bonds as it need 2 p orbitals
- Alkenes general formula ?
-
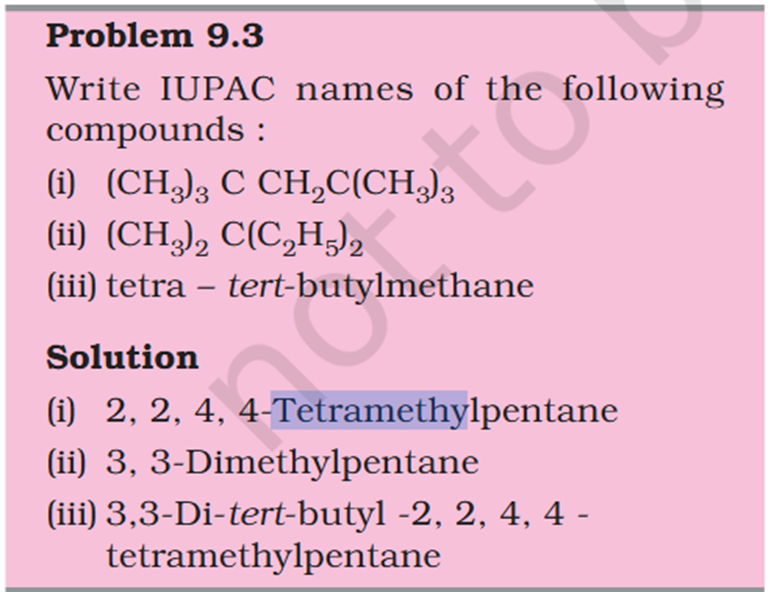
[Organic Chemistry] Nomenclature
Chloromethyl ? C3H5 what is that ? 1. Supposed to be CH3Cl which should be Chloromethyl Ethyl Propyl
-
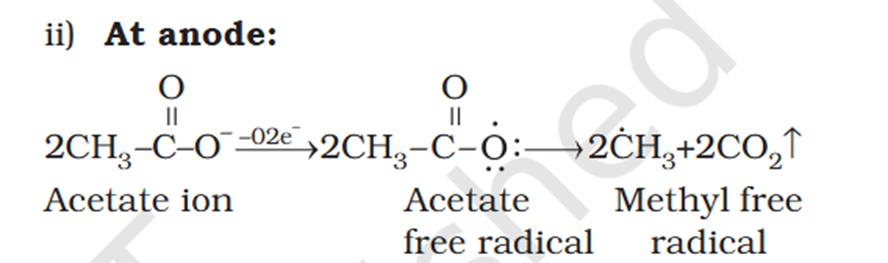
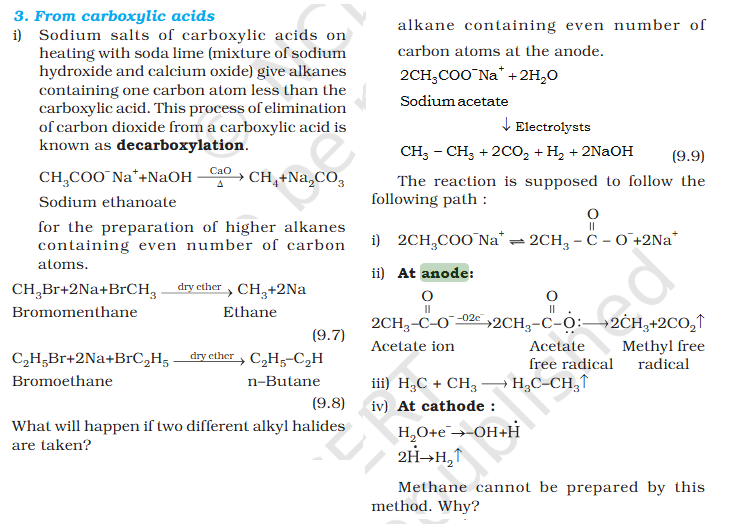
[Organic Chemistry] Explain decarboxylation
What is acetate ? not methyl carboxylic acid Why is O negatively charged oxygen has 6 electrons and when bond it's got 7 if it losses 2 what is 5 but the middle part has 7 electrons oxygen has 6 electrons and when bond it's got 7 if it losses 2 what is 5 but the middle part has 7 electrons oxygen has 6 in valence free radicals has single electrons in their orbital in the valence shell, making atom unstable, reactive, shifts electron cloud Reaction is from
-
[Organic Chemistry] Nomenclature
5-isopropyl Iso means 1 branch of methyl on 2nd Propyl is c2h4 not 6 Sec-butyl is c with 2 other c attache and another fcitonal group attached Also iso thing Propy is c3h8 not 6 Here propyl mean C3H5
-
Organic Chem: Dumas method
@exchemist sorry struggling with thermodynamics
-
Organic Chem: Dumas method
How does this conversion multiplication works ? @exchemist Dhanyavad
-
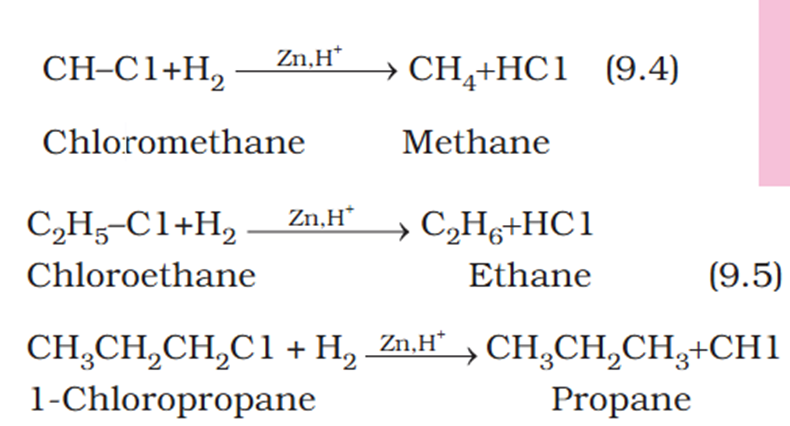
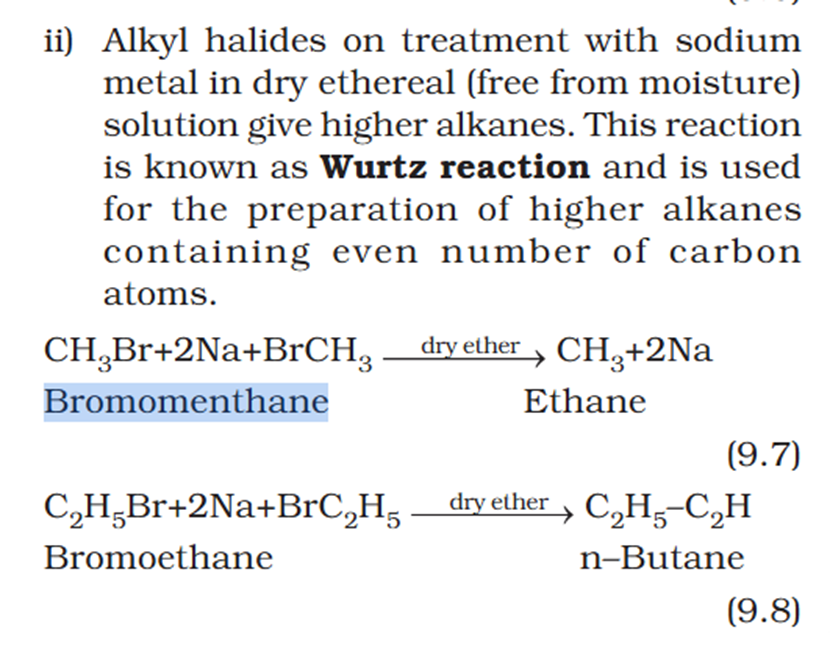
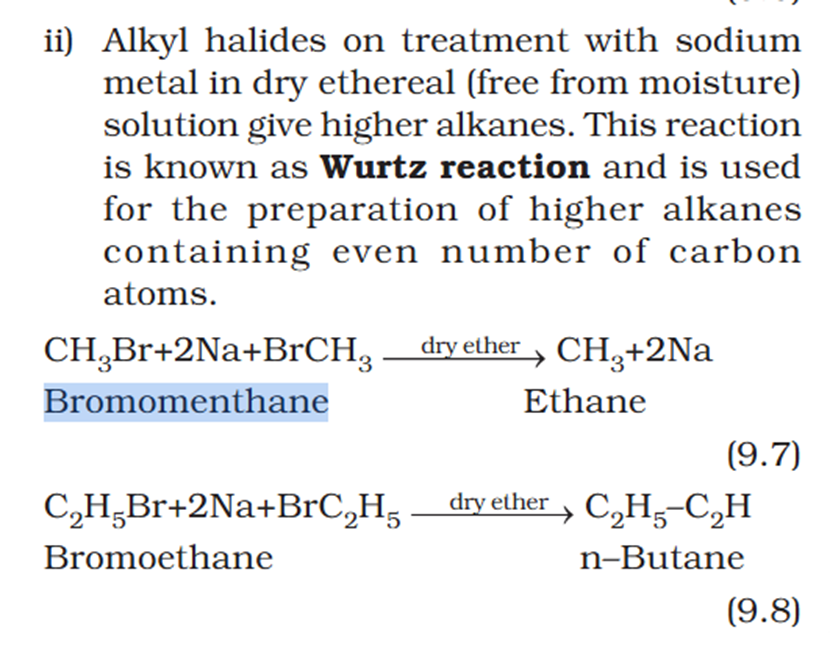
Organic chem explain Wurtz reaction.
- Organic Chem: Nomenclature
5-isopropyl Iso means 1 branch of methyl on 2nd Propyl is c2h4 not 6 Sec-butyl is c with 2 other c attache and another fcitonal group attached Also iso thing Propy is c3h8 not 6 Here propyl mean C3H5 What is tetra What is tert Chloromethyl ? C3H5 what is that ? 1. Supposed to be CH3Cl which should be Chloromethyl Ethyl Propyl What is acetate ? not methyl carboxylic acid Why is O negatively charged oxygen has 6 electrons and when bond it's got 7 if it losses 2 what is 5 but the middle part has 7 electrons oxygen has 6 electrons and when bond it's got 7 if it losses 2 what is 5 but the middle part has 7 electrons oxygen has 6 in valence free radicals has single electrons in their orbital in the valence shell, making atom unstable, reactive, shifts electron cloud Reaction is from- Organic Chem: Dumas method
@sethoflagos @exchemist thank you In standard gibs energy 25C is used are STP. what does multiplying with 760 and 273 achieve. @sethoflagos sorry sometimes I take time to reply and my English is good, that is Arabic for thank you you wrote not any Indian language 😀- Redox Reactions: What takes priority in findng which Element is Oxidized - Add/Remove of H/O, Electron transfer, Electronegative/Positive element ?
@exchemist thanks alot- Organ Chem: Is C-C bond length same in C2, C2H4, C2+Halogen or other elements ?
Double bond - C2H4 should be longer than C2. And if a more electro negative is added to C2H4 single bond or C2H6 double bond, length should reduce further. - Organic Chem: Nomenclature
Important Information
We have placed cookies on your device to help make this website better. You can adjust your cookie settings, otherwise we'll assume you're okay to continue.