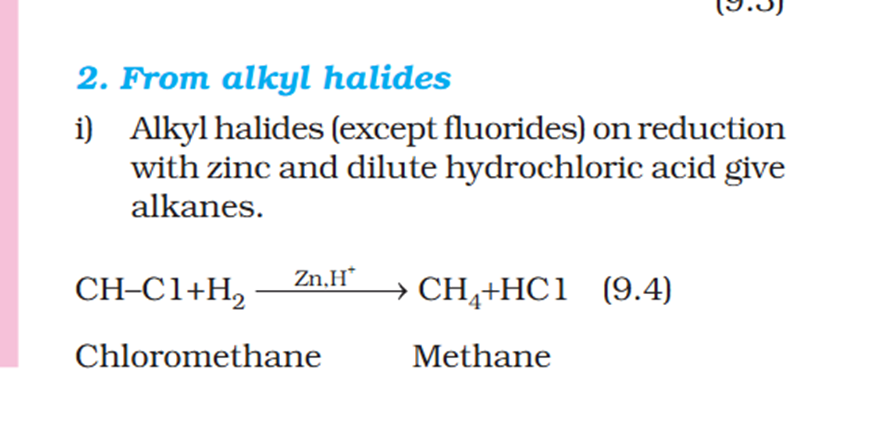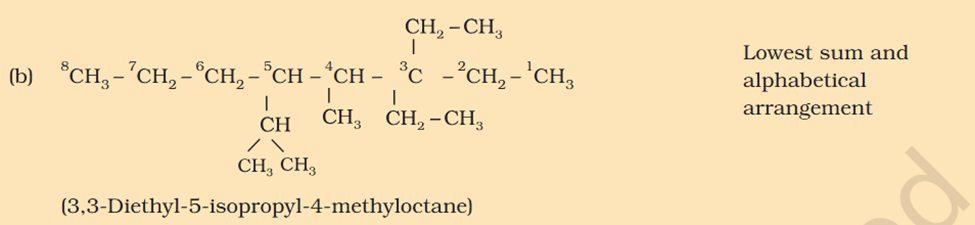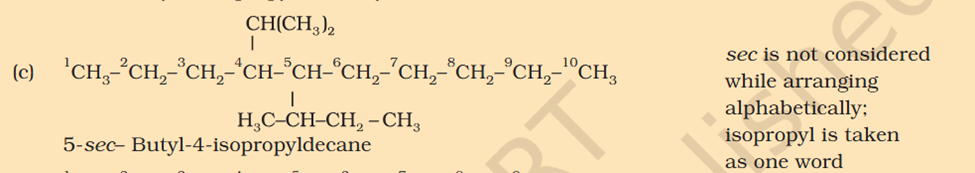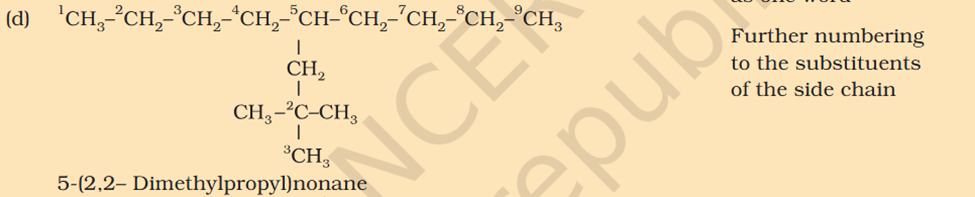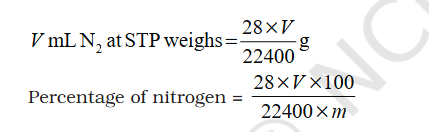Everything posted by HbWhi5F
-
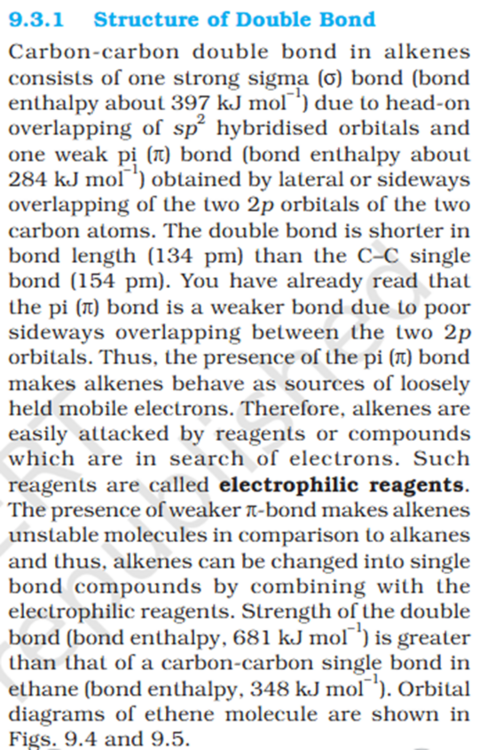
Pie bond uses 2 p orbitals ?
'You have already read that the pi (π) bond is a weaker bond due to poor sideways overlapping between the two 2p orbitals." 1 pie bond is actually 2 bonds as it need 2 p orbitals
- Alkenes general formula ?
-
[Organic Chemistry] Nomenclature
Chloromethyl ? C3H5 what is that ? 1. Supposed to be CH3Cl which should be Chloromethyl Ethyl Propyl
-
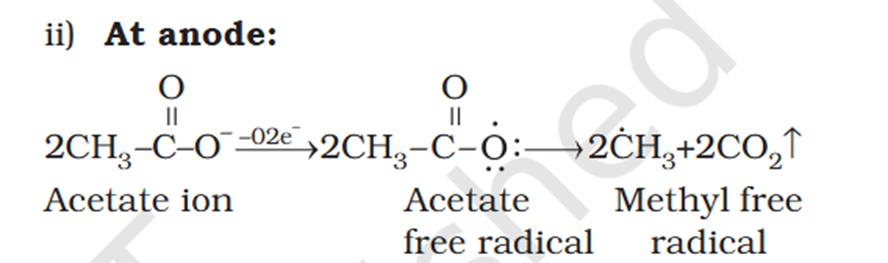
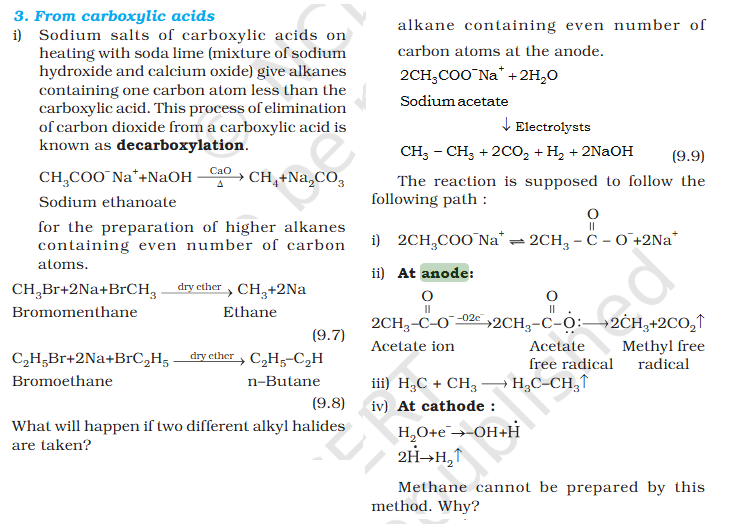
[Organic Chemistry] Explain decarboxylation
What is acetate ? not methyl carboxylic acid Why is O negatively charged oxygen has 6 electrons and when bond it's got 7 if it losses 2 what is 5 but the middle part has 7 electrons oxygen has 6 electrons and when bond it's got 7 if it losses 2 what is 5 but the middle part has 7 electrons oxygen has 6 in valence free radicals has single electrons in their orbital in the valence shell, making atom unstable, reactive, shifts electron cloud Reaction is from
-
[Organic Chemistry] Nomenclature
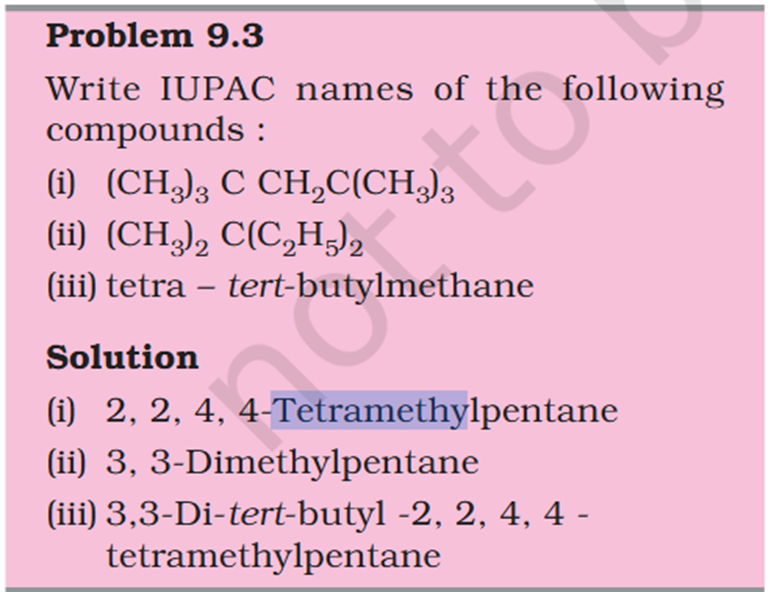
5-isopropyl Iso means 1 branch of methyl on 2nd Propyl is c2h4 not 6 Sec-butyl is c with 2 other c attache and another fcitonal group attached Also iso thing Propy is c3h8 not 6 Here propyl mean C3H5
-
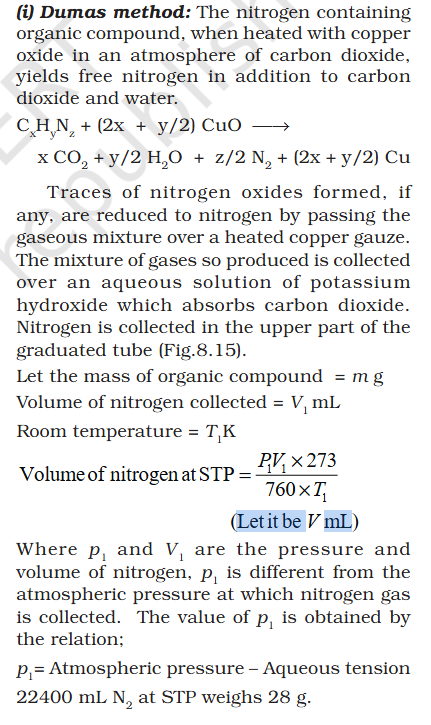
Organic Chem: Dumas method
@exchemist sorry struggling with thermodynamics
-
Organic Chem: Dumas method
How does this conversion multiplication works ? @exchemist Dhanyavad
-
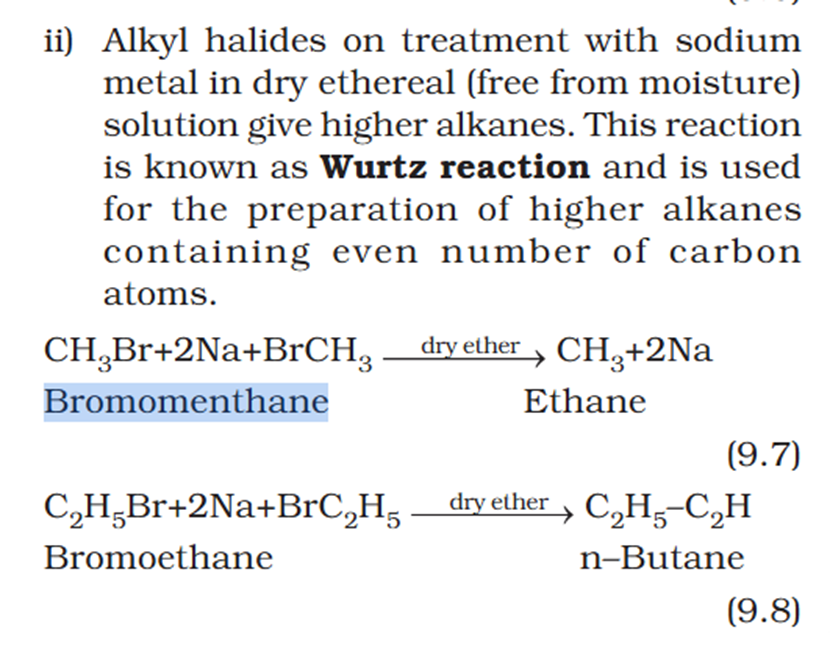
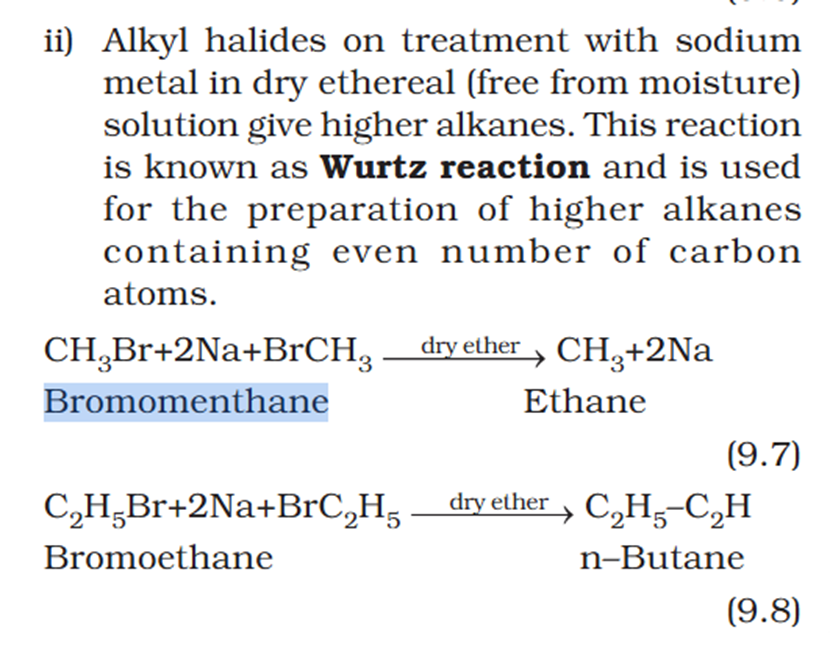
Organic chem explain Wurtz reaction.
- Organic Chem: Nomenclature
5-isopropyl Iso means 1 branch of methyl on 2nd Propyl is c2h4 not 6 Sec-butyl is c with 2 other c attache and another fcitonal group attached Also iso thing Propy is c3h8 not 6 Here propyl mean C3H5 What is tetra What is tert Chloromethyl ? C3H5 what is that ? 1. Supposed to be CH3Cl which should be Chloromethyl Ethyl Propyl What is acetate ? not methyl carboxylic acid Why is O negatively charged oxygen has 6 electrons and when bond it's got 7 if it losses 2 what is 5 but the middle part has 7 electrons oxygen has 6 electrons and when bond it's got 7 if it losses 2 what is 5 but the middle part has 7 electrons oxygen has 6 in valence free radicals has single electrons in their orbital in the valence shell, making atom unstable, reactive, shifts electron cloud Reaction is from- Organic Chem: Dumas method
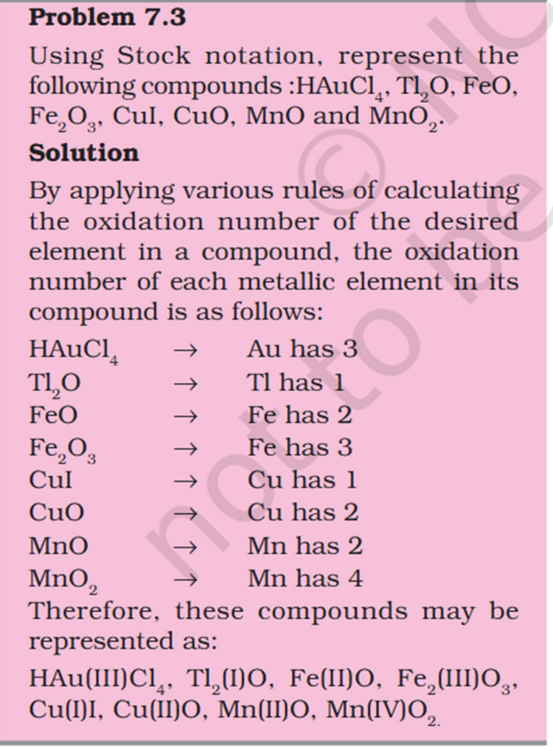
@sethoflagos @exchemist thank you In standard gibs energy 25C is used are STP. what does multiplying with 760 and 273 achieve. @sethoflagos sorry sometimes I take time to reply and my English is good, that is Arabic for thank you you wrote not any Indian language 😀- Redox Reactions: What takes priority in findng which Element is Oxidized - Add/Remove of H/O, Electron transfer, Electronegative/Positive element ?
@exchemist thanks alot- Organ Chem: Is C-C bond length same in C2, C2H4, C2+Halogen or other elements ?
Double bond - C2H4 should be longer than C2. And if a more electro negative is added to C2H4 single bond or C2H6 double bond, length should reduce further.- Organic Chem: Dumas method
- Organic Chemistry - Resonance Structure and Electrometric Effect: Need help conceptualizing
-ve O has 8 electrons so it's -2. +O has 8 electrons too and why it's negative.- Redox Reactions: What takes priority in findng which Element is Oxidized - Add/Remove of H/O, Electron transfer, Electronegative/Positive element ?
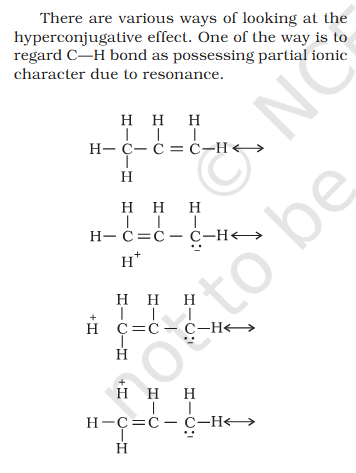
@exchemist In (i) What takes priority Electron transfer or Electronegative/Positive element ? I think more electronegative is a good oxidant In (i) Explain addition of Electronegative/Positive element seen as oxidation When the other atoms is more electronegative it is oxidized In (iii) How can Na be oxidized when hydrogen is been added to it ? This part is wierd hydrogen being added is reduced.- Organic Chemistry Hyperconjugation Resonance: Partial +ve charge on H and neutral C, role of -ve charge on C ?
It seems like a miss-print/render H+ is partially positive but still bonded ? Positive charge needs to be on C for it's empty p-orbital to be stabilized by adjacent C-H bon's sigma bond. What is the role of -ve charge on C ?- Redox Reactions: What takes priority in findng which Element is Oxidized - Add/Remove of H/O, Electron transfer, Electronegative/Positive element ?
Problem 7.1 In (i) What takes priority Electron transfer or Electronegative/Positive element ? In (i) Explain addition of Electronegative/Positive element seen as oxidation In (iii) How can Na be oxidized when hydrogen is been added to it ?- Organic Chemistry - Resonance Structure and Electrometric Effect: Need help conceptualizing
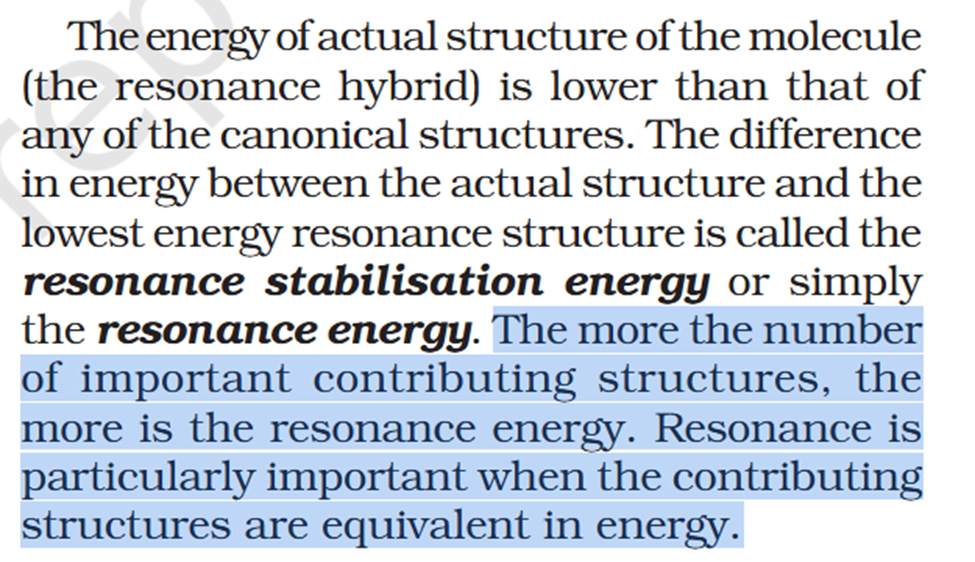
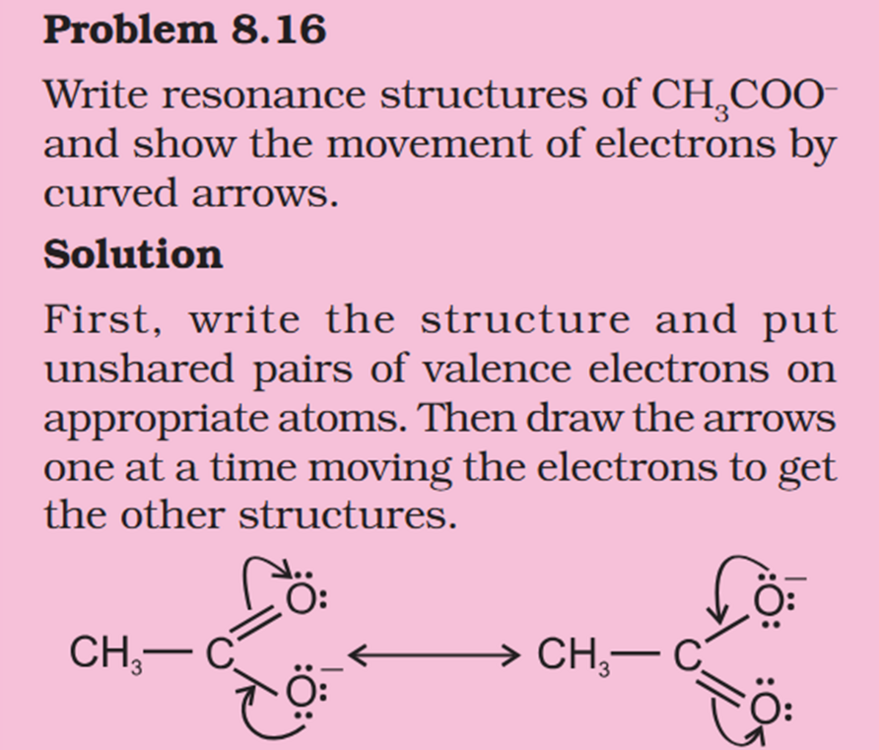
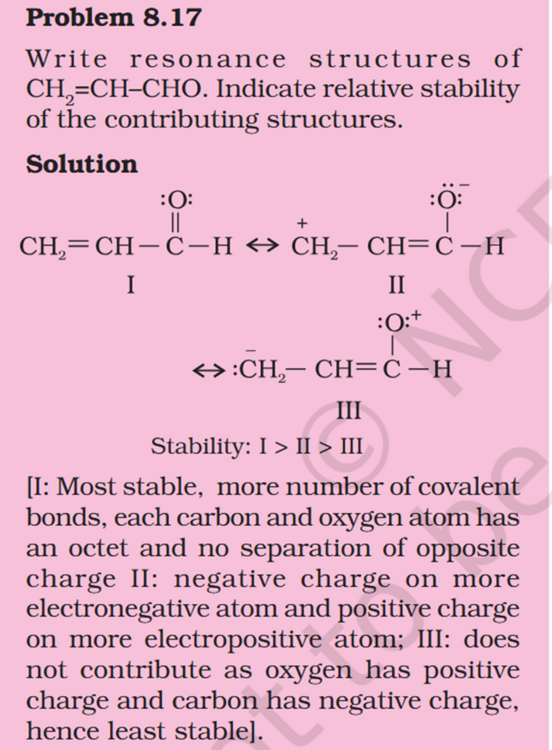
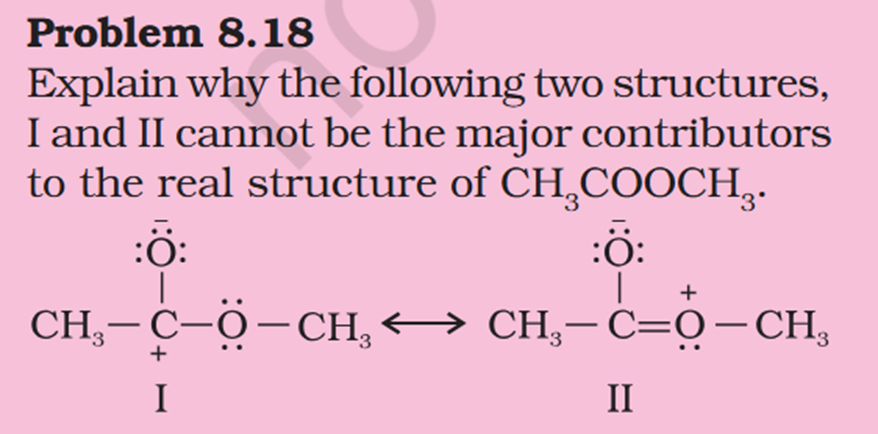
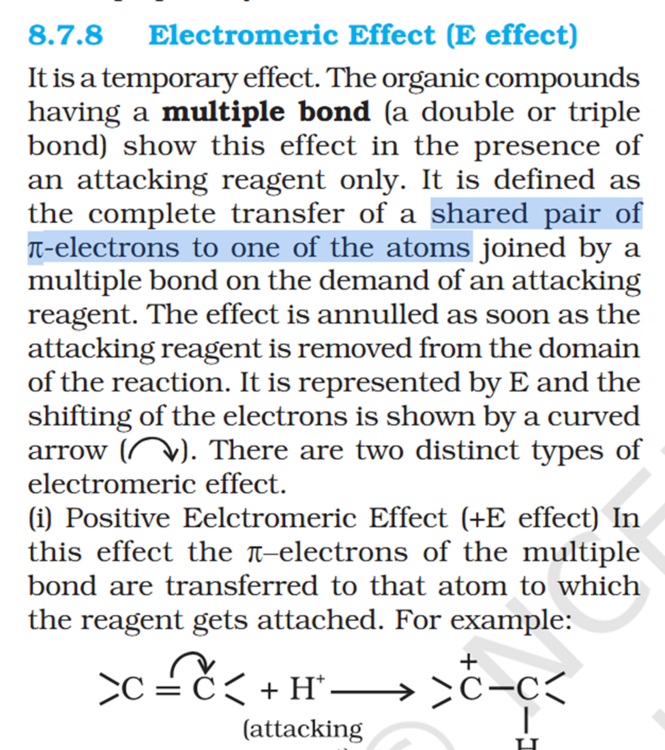
Concept If resonance energy is more that means the means resonance structures have varying energies and it should be less stable aka more energy ? So more resonance energy = More Stability ? Problem 8.16 Does the electron movement be of 1 electron and not a pair. C needs 4 more electrons the 2 hooked arrow shows C is getting 1 extra electron. Problem 8.17 Resonance structure needs to have same no. of unpaired electrons. Why charges develop of II Why is O in III +ve Explain “does not contribute as oxygen has positive charge and carbon has negative charge, hence least stable].” Problem 8.18 In II why would O develop +ve charge Explain the answer Electrometric Effect Book says transfer to Pie bond but pie bond atom always looses- 4-Ethyl-2-methylaniline: Why is C2H5 called Ethyl ? and Alternative names ?
Actually 1 wrote "It would be good if some of your countrymen would step in here and tell you that it doesn't hurt to respond to those who have genuinely put themselves out to help you." Many be I am reading it wrong but what is "countrymen" about ?- Why assume hybridization and not charges on Carbon ?
@exchemist @pinball1970 Sorry if i haven't thanked you for your answer but I do read them, it isn't like I just post question if you feel that way.- Is Pie bond of Double and Triple Bond CC different ?
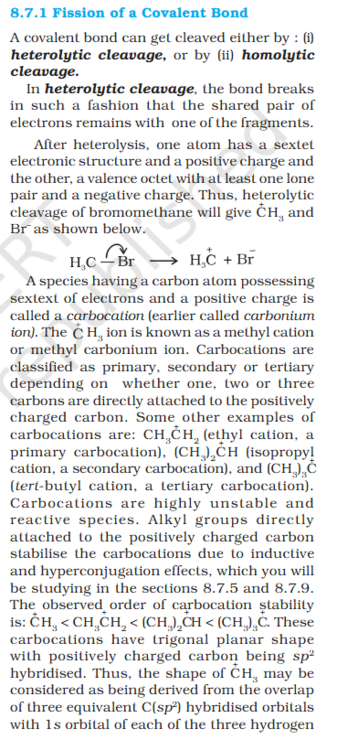
@studiot thanks @exchemist- Organic Covalent Bond Fission: Why it's assumed that +ve ion has a sextet and other 10 electrons ?
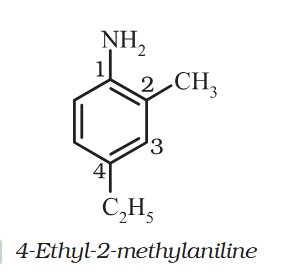
- 4-Ethyl-2-methylaniline: Why is C2H5 called Ethyl ? and Alternative names ?
@KJW Is there a parent compound priority list? Also thanks for the reply I am being boycotted on the forum. I know I take sometime to reply but somehow that make fellow scholars racist.- 4-Ethyl-2-methylaniline: Why is C2H5 called Ethyl ? and Alternative names ?
@KJW I understand 4-Ethyl-2-methylaniline is the correct name but Is 1-amino-4-Ethyl-2-methybenzene wrong ?- 4-Ethyl-2-methylaniline: Why is C2H5 called Ethyl ? and Alternative names ?
Ethyl is C2H6. Why why C2H5 is called that here ? Aniline means benzenamine (C₆H₅NH₂) but NH2 is at 1. So it can also be written as 1-amino-4-Ethyl-2-methybenzene ? - Organic Chem: Nomenclature
Important Information
We have placed cookies on your device to help make this website better. You can adjust your cookie settings, otherwise we'll assume you're okay to continue.