Posts posted by Gian
-
-
22 hours ago, studiot said: Your graphic states "and are then converted into nitriles"...
7 hours ago, exchemist said: Exactly. See my later post and the link to the paper. It’s pretty complicated but you can see the sort of thing that goes on.
11 hours ago, sethoflagos said: The strength of the CN triple bond in nitriles ...
Dear Messrs Exchemist, Studiot & Sethoflagos
I've had this from Dr Lorenz, and although it's mostly above my head I think I can follow it enough so I'll use this in my graphic,Lorenz, Ralph D.<Ralph.Lorenz++++++>
Gian
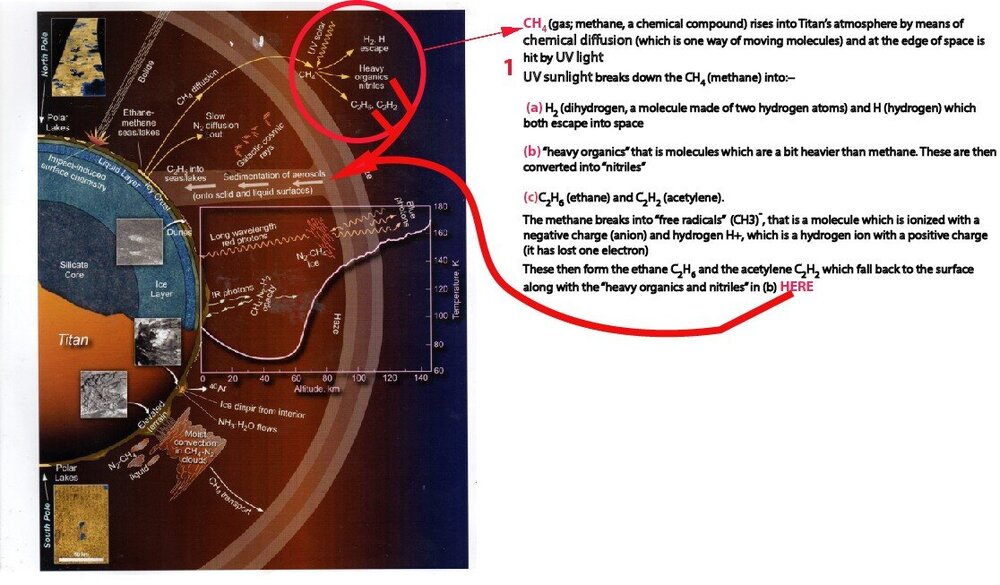
You’ve almost got it, but strictly it’s like this (I would say your wording ‘heavy organics.. .converted into nitriles’ is not quite correct)
UV & electrons break CH4 into bits - CH, CH2, CH3, H
Electrons also break N2 into bits N
These fragments can recombine (the following equations sum up what could actually be several steps, which may or may not involve excited states, ions/electrons or third molecules that soak up energy, but are simplified for explanation)
H + H -> H2
CH + CH -> C2H2 (acetylene)
CH3 + CH3 -> C2H6 (ethane)
N + CH2 + CH -> C2H3N (acetonitrile)
And so on to C6H6 (benzene) and many more complicated hydrocarbons (C,H compounds) and nitriles (C,H,N compounds)
The H2 escapes to space. Everything else eventually ends up on the surface
Ralph
Hope this makes sense to everyone and additional comments appreciated
CheerzGIAN🙂XXX
-
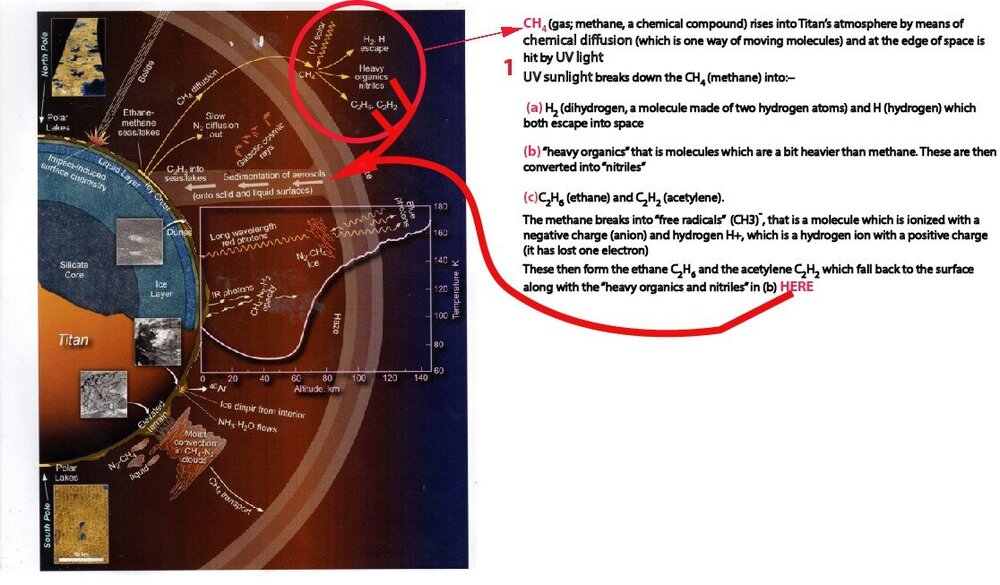
2 hours ago, exchemist said: Regarding your ( c ) , if a free radical is formed it will be CH₃• rather than CH₃⁻, and the hydrogen released will be H• rather than H+. The dot denotes an unpaired electron, which makes the species reactive. There is no charge separation in free radical reactions so both species are uncharged. CH₃• is known as a methyl radical. A pair of these certainly combine to give ethane, C₂H₆. However the formation of acetylene, HC≡CH, would need further explanation. I could imagine methyl radicals removing H from ethane, perhaps, but 4 would have to be removed before you reached acetylene so this route seems a bit doubtful. Is there a further explanation in Lorentz's book for how acetylene is formed?
Thanks, so the CH4 (methane) doesn't change into an an anion, but into CH₃• which is a "methyl radical?"🙂
-
19 minutes ago, studiot said: Sorry I have no further information at the moment, you will need to look it up.
Perhaps @exchemist or @sethoflagos or @chenbeier might add more.
Thanks, I've also contacted Prof Lorenz, but I wanted everyone here's take too🙂
-
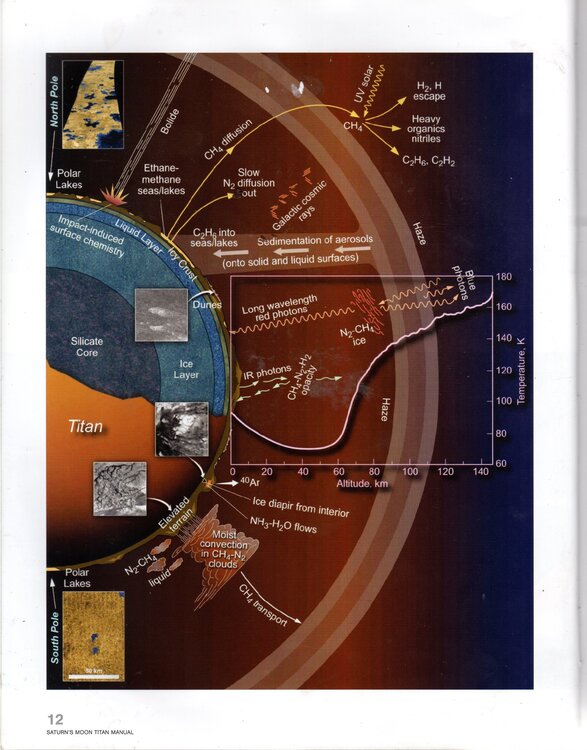
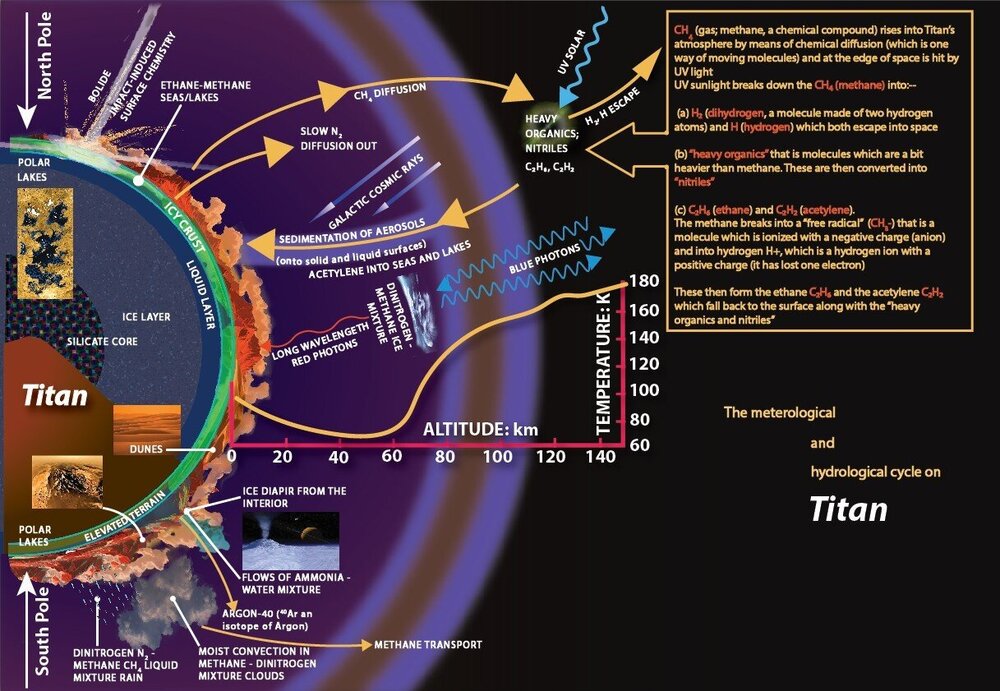
I've done my own version of a diagram of Titan's hydrological cycle (attached,) the original (also attached) being on p12 of Dr Ralph Lorenz's great book Saturn's Moon Titan: Owners' Workshop Manual. Can anyone take a look and see if I've got it right please? Im particularly concerned about what I've put in the big text box about what happens to methane diffusion when it's hit by UV rays.
Looks like H2 and H escape to space, and other components are broken down and fall back to the surface, but I'm not sure.
It's for a graphic design project
Cheerz
GIAN🙂XXX(SCIENCE AGE ABOUT 12)
-
-
Edited by Gian
12 hours ago, swansont said: Where was this suggested?
https://www.sciencedirect.com/science/article/pii/S0277379120302936?via%3Dihub
It suggests that "assimilation'' played only a small role, nevertheless the role was there, but haven't read the whole paper yetGIAN🙂XXX
-
If we were going to terrafotm Venus, besides cooling her down and giving her a breathsble atmosphere and a magnetic field, I guess we'd need to speed up her incredibly slow rotation to give a 24h day.
I've read that this vould be theoretically achieved, if 2 or 3 asteroids about 350km across were fired at Venus in quick succession, presumably from the asteroid belt.
But it's difficult to see how such huge bodies could be moved.
However if a load of small asteroids with nuclear rocket engines all crashed together at 1 particular point, and at the right trajectories, could the resultant lump of rock and debris then speed towards Venus to do the job? A bit like snooker?
Cheerz
GIAN🙂XXX
(science age 12¾)
-
It's been suggested that our neanderthal cousins disappeared because they "assimilated" with our remote ancestors within our subspecies, homo sapiens sapiens. Is this right?
I assume that means they interbred. If so, how can they be called a separate species? Surely they're the same as us, just a different ethnic group
CHEERZ
GIAN 🙂XXX
(science age 12¾)
-
-
Edited by Gian
On 5/10/2025 at 3:50 PM, raphaelh42 said: Hi
i understand that no matter if we want:
a ton of money to buy cool cars
make the world a cleaner place
help animals
We do this because achieving it would make us happy...
Depends what you mean by "happy." The way to real happiness is not as it were to be happy, that's quite easy.
The way to happiness is to be useful
So yes, helping animals and conserving our planet are both very useful, as is looking out for your neighbours, supporting the homeless or helping an old lady across the roadCheerz
GIAN🙂XXX -
-
-
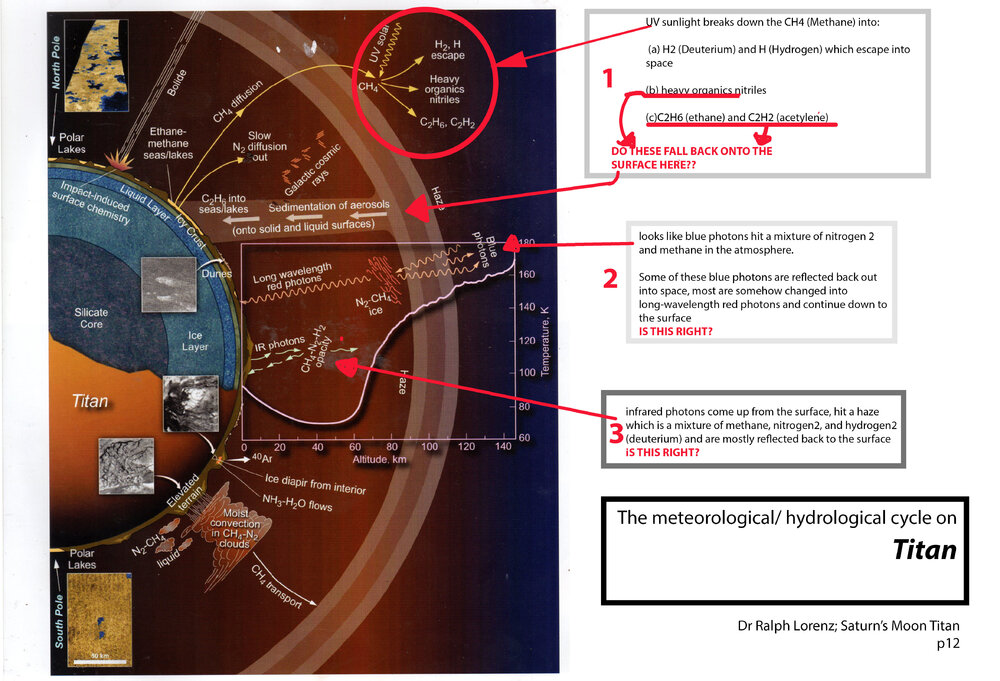
On 5/9/2025 at 7:38 PM, swansont said: #2 Yes. When a molecule absorbs a photon that excites it above the lowest excited state it can then emit lower-energy photons as it decays back to the ground state
On 5/9/2025 at 9:05 PM, exchemist said: Re yr 1, I would think that yes, since the temperature at the surface is below the boiling point of ethane and acetylene, these compounds will fall out of the atmosphere. As “rain”, maybe?
Re yr3, yes methane is a greenhouse gas so will absorb and re-radiate in the infra red. Nitrogen and hydrogen however will be transparent to IR, having no dipole in the molecule.
On 5/9/2025 at 8:38 PM, studiot said: Whilst I can find quite a details of Titan's atmousphere in
Thermodynamics of the Earth and Planets - Alberto Patino /douce - Cambridge University Press
I cannot find any reference to heavy hydrogen (deuterium) so
I would like to clear up a clear up what may be some misconception.
1
Deuterium is also called heavy hydrogen (as in heavy water) because it is ordinary hydrogen with an extra neutron in the nucleus. It's symbol is not H2, ( It is D) which is the hydrogen molecule made from two ordinary hydrogen atoms.
None of the compounds named in (b) and (c) are really heavy organics, they are just a bit more massive molecules than methane.
But yes, sunlight (UV) and not so strong at Saturn's distance from the Sun breaks down (dissociates) the methane into 'free radicals' (CH3)- and H+.
These are highly reactive and so two mehtyl radicals can join together to form acetylene or one methyl and one methane molecule to form ethane, loosing an H- which joins with the other H+ to form a hydrogen molecule.
2 See swansont's answer
3
Yes I understand there is an outer haze that forms which acts in the same way as the earth's greenhouse effect by reflecteing back the longer radiation from the surface (although I'm not sure if this is now longer than infra red), whilst permitting passage of shorter wavelengths in the sunlight.
Hi Mr Swansont, Mr Studiot and Mr Exchemist
I've done an amended version of the graphic I showed you (re the first bit) Can you all have a look and tell me if it reads ok please?
Mr Studiot thanks for the paper you sent me, can't quite fathom it at present but I'm working on it!
ThanksGIAN 🙂XXX
science age; 12 (pre-gcse)
-
-
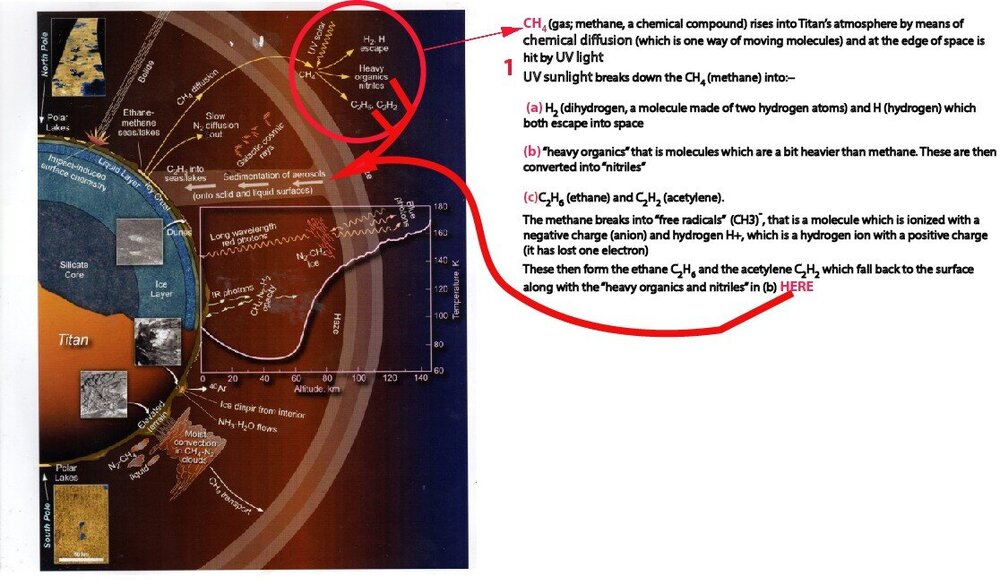
In his book Saturn's Moon Titan; A Workshop Owner's Manual Dr Ralph Lorenz has a graphic describing Titan's meteorological/ hydrological cycle, and also a chapter. I've attached a scan of the graphic with 3 questions. Can anyone give me a hand with my 3 queries please about methane diffusion, blue and red photons and infrared photons?
Re query 1, at the top of the diagram it looks like Methane rises into the atmosphere and then "diffuses" into other chemicals
ThanksGIAN🙂
science age 12 (pre-GCSE)
-
On 1/18/2024 at 11:37 PM, Ant Sinclair said:
I agree there has to be "something" and it is impossible for "something" never to have existed, but, I also believe "nothing" has existed. Something came from Nothing, but how?
Not quite sure if this is relevant, but St Thomas Aquinas described God not as the creator but actus essendi ; which (I think) translates not as "a being" but "the act of being" or "the act of existence"
If so then this is an abstract and I guess doesn't need to be created. It just is. -
Don't know if this should be in the PHYSICS section or not
I'm a digital animation student.
I want to generate a video of what happened inside the passenger cabin of the Boeing 747, The Maid of the Seas (Pan-Am Flight103) when it exploded over Lockerbie Scotland at 31,000ft.
What sort of physics would I need to map the event in realtime?
1. I've read that in a few milliseconds the explosion in the hold punched a 20" hole in the fuselage. This would cause an almost instant decompression of air exiting through the hole. Wikipedia says 0.1-0.5 seconds. Mathematically, how do I calculate exactly how fast in microseconds?2.There are vents lining the cabin walls of an airliner (dado vents, image attached) which open to the hold in the event of a decompression in the hold, to prevent the floor collapsing.
The investigators say the bomb did not disrupt the cabin floor. In the first few milliseconds, presumably everything in the cabin not tied down and above a certain weight would have flown towards the vents with the speed of debris from a grenade going off, but as an implosion. Is this right?
3. How to I work out how fast unsecured objects would fly towards the vents in the implosion? Could it carry a human being not strapped in his seat? In the video simulation attached it looks like it would be
4. Would the speed of objects carried by the implosion vary according to weight?5. How would I work out if the implosion would be powerful enough to tear passenger seats from their moorings and towards the implosion?
I guess I'll need among other things I'd need the air density of air at 31,000ft, the air density and volume inside the cabin and the number and size of the dado vents.
This is about just in the first few milliseconds (I'll get to the physics of what happened when the fuselage broke apart later)
Meanwhile I'm searching the net. Any ideas? Can you point me to anything I can read? Any guidance gratefully received
CheerzGIAN🙂XXX
DECOMPRESSION SIMULATION:
https://youtu.be/EHGBQINW0B0
DADO VENT:
-
The BBC's GCSE Chemistry page says there are eight 8 groups in the Periodic Table; 1-7, plus 0 (noble gases?) But there are ten columns between 2 and 3 with no number:
https://www.bbc.co.uk/bitesize/articles/zptfn9q#zkn27yc
However, other versions of the Table like this one from the The Royal Society of Chemistry show 18, eighteen groups. What's going on??
https://www.rsc.org/periodic-table
I should emphasise that age 32 I'm pre-GCSE level so my science age is about 12½ please keep answers as simple as possible
Cheerz
GCSE GIAN😊XXX
science age 12½ -
13 hours ago, studiot said:
I seriously suggest you start a new thread to ask about bonding.
There will be plenty of help there available and worthwhile descriptions can be offered at your level.
Remember bonding is a Chemistry subject, so you should post there.
Thanks, will do!
"GCSE GIAN" 🙂XXX
science education age about 12½
-
Hi Gents, I'm not studying GCSE science (yet) but what I mean is I'm at about that level. But I've started using the GCSE syllabus as a guide, which I think is a sensible place to start. Although I also can't help looking at A level stuff, and stuff written by Prof Brian Cox et al.
It's easy to get disheartened with stuff that's way over my head. I don't think I'm going to get this quantum "spin thing" at this stage, but I've just gotta tell myself I will get it one day
However I think I'm getting on well with the structure of the atom, elements, compounds, molecules and mixtures. About to move onto ionic bonds covalent bonds.
Thanks for all the input
cheerz"GCSE GIAN" 🙂XXX
science education age about 12½
-
54 minutes ago, swansont said:
Spin with a charged particle gives them a magnetic moment, so “spin up” and “spin down” (the two possible values of the spin orientation) will have a different energy in a magnetic field, which you have in an atom.
So what is a "magnetic moment?"
cheerz
GIAN🙂XXX
science education age about 12½
-
15 hours ago, exchemist said:
OK. Like a lot of things in quantum theory this is not like the behaviour we are used to at the scale of everyday life, so it may take a few iterations to understand it.
15 hours ago, studiot said:Remember the spin quantum number is responsible for the magnetic properties of the substance.
Thanks guys, well I don't think I'm going to get it this time, other than it sounds like it's like potential energy, wrapped up in the electron.
I'm still at GCSE level maths and physics and quantum theory doesn't seem to come into it at this stage. Maybe it will become clearer when I've done a lot more stuff first.
cheerzGIAN🙂XXX
science education age about 12½
-
-
Can't get my head around this.
"Spin is the intrinsic angular momentum of particles. Spin is given in units of ħ which is the quantum unit of angular momentum where ħ = h/2π = 6.58x10-25 GeV s = 1.05x10-34 J s"At this stage I just want to know;
1 When it says "spin" is the "angular momentum," does it mean the speed that these particles (Fermions and Bosons?) rotate at, or what?
2. What's the h in the equation h/2π = 6.58x10-25 ??
3. Is the funny symbol ħ to do with something called the planck constant?
Cheerz
GIAN🙂XXX
science education age about 12½

Epidermolysis bullosa (EB): PAIN BUT NO GAIN
in Medical Science
There's no cure for Epidermolysis bullosa (EB,) apparently it's a genetic thing.
But does anyone know what a cure would look like?
Thanks
GIAN🙂XXX
(science age about 12)
https://en.wikipedia.org/wiki/Epidermolysis_bullosa#Pathophysiology