-
Posts
534 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by DQW
-
-
Really, there's not much to be understood there. All I'm saying is that our understanding of neutrinos has changed considerably since after Smolin wrote his book.
The rest is just keywords you can Google for more info on the subject.
0 -
There are two broad questions here, that I'll address briefly :
1. How is reflection any different from fluorescence (absorbtion and re-emission) ?
Fluorescence, is an atomic phenomenon, while reflection is not. By that, I mean that (i) fluorescence involves excitations and decays within the electronic levels of individual atoms, while
(ii) reflection involves collective excitations of either an electron gas (in metals, known as plasmons) or of phonon modes (in insulators and semiconductors, these are the optical phonon modes).
Simplistically speaking, (in the case of reflection) the incident photon excites a collective oscillation in the free electron gas/lattice. These oscillations are unstable and decay rapidly, re-emitting the photon that excited them. These excitations conserve momentum, making reflection conserve angle.
2. Why does the emission spectrum of a fluorescent material always look "down-shifted" from the excitation spectrum ?
This is simply due to the large number of energy levels in any real material. An excitation between a pair of levels allows for a large number of possible decay modes (see pic below for an example showing 3 such modes). The emission spectrum is simply the sum over all these decay spectra (weighted by their probabilities). Clearly the maximum energy of a single transition to the ground state will be the excitation energy itself. In addition to this, several other intermediate energies (and hence photon frequncies) are sampled through the different decay modes.
It is this breaking up that causes the "down-shift" in the emission (aka decay) spectrum, compared to the excitation (aka - though loosely - absorption) spectrum.
0 -
Comments on above geometry :
1. The advantage of the geometry is that it will increase stability intrinsically (ie: if it were in air)
2. The drawback (over a two piece system with the SC on the bottom) is that you now have to have liquid N2 all the way up to the SC (submerging it completely). The vigorously boiling liquid will definitely introduce instabilities which may knock the SC out.
3. The geometry is dependent on the dimensions of the parts. What are the shapes and sizes of the SC and the magnets ?
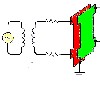
No, there won't be a shielding effect. See attached pic.
0 -
Never heard of an "electron-specific Higgs particle".
In any case, you should not believe very much in Smolin's book when it comes to neutrinos. The book was published in 1997, about a year before the SuperKamiokande group announced a non-zero lower bound on the neutrino mass.
Current understanding of neutrino mass (and its incorporation into the Standard Model) comes from the picture based on Majorana neutrinos (and also involves interaction with a Higg's Boson, to acquire mass), if I'm not mistaken.
0 -
You will see the "and" in most literary usage :
One Hundred and one Dalmatians, One Thousand and One (Arabian) Nights, etc.
0 -
I would start from the right side and use the half-angle formulae instead.
0 -
I can see how an "and" might imply an fractional part. But a decimal point ? Wouldn't you just say "point", as in for the number 4.3, you would write "four point three" rather than "four and three" ?Actually I don't think the 'and' is supposed to be there, because 'and' implys a decimal point. It would just be "one thousand one"Anyway, Mathworld confirms that using "and" is okay.
http://mathworld.wolfram.com/Number.html
The hyphenation of numbers from twenty-one to ninety-nine is important though (if you want to be all proper and such).
 0
0 -
I've seen a picture of a car hanging of such a permanent magnet (no, it was not an electromagnet).I'm in York County - about 10 miles away from Jlab. The last unusual material, which wasn't directly for JLab, but which ended up there, was a super magnet. The company which was doing the experiment forgot to order one with a hole in it. You probably know those magnets are sintered, so they shouldn't be so difficult to cut - had we been able to get the chips out of the hole! I had never seen a "super magnet" before - you could not pick it up from magnetic material, I don't know how much strength it would take to make one release its hold on an iron table, but one of my employees who is a weight lifter, could not budge it. We had to slide it onto a piece of plastic.
Wow, that's interesting ! I wouldn't have thought that these would have been really hard to remove. These magnets are usually magnetized in the direction of the thickness (not along the length or width/dia - in which case it would be impossible to remove the chips).Anyway - when we tried to drill the hole the chips adhered to the inside of the hole as though the material had never been drilled.
How big was this magnet ? I'd be willing go bet a smallish sum that this was a sintered NdFeB magnet. Those things are quite brittle. The first thing you are warned about, when handling NdFeB magnets is to be very careful when you are near metal or when handling two or more magnets. They accelerate (if released) rapidly towards anything iron or steel and strike it hard and shatter into tiny pieces. But this is more true of the smaller sized magnets.but I We asked the fellow how much it would cost to reorder the magnet with the hole, and based on the price, told him it would be more cost effective to do that. I'm sure we could have figured out something eventually, but not for $40 or $50.I'm surprised you were able to drill it easily - they're almost like ceramics, in terms of machinability. Are you sure it didn't have some kind of epoxy-based binder ? Those are called bonded NdFeB magnets, and are much easier to machine than the pure, sintered kind. But the sintered magnets are stronger (magnetically).
Did you have to use diamond bits ? I'm sure you were warned to used lots of coolant (the magnet will demagnetize at about 250F), and to be prepared to handle a fire. The chips are extremely flammable (like magnesium powder). If they get too hot, they will auto-combust and burn with a bright, hot flame making all kinds of toxic gases.
17-4 for high yield strengths I guess. I was imagining a lot of high-vacuum parts for the accelerator, for which you would need non-magnetic steels. The austenitic steels (like 304) are much better in this respect, than the martensitic steels (like 17-4PH).We use 17-4 as the main material for machines we build which repair high pressure steam valves in-line on nuclear vessels. It cuts reasonably well and does not change significantly after heat treating - therefore, you don't have to grind it.
That is neat ! I remember the news about the dolphins - I thought it was in the Mediterranean, but I must be mistaken.Another neat job we did was "pinger housings". Do you remember hearing that dolpins were used to find mines in the Red Sea? We built the metallic portion of the parts that trained them. The explosives were removed and parts that emitted a particular "ping" were substituted. The dolphins were trained to find them by the noise they emitted and then trained to recognisz the mines on sight.
I found this extremely bothersome when I wanted to machine some teflon in a hurry and had a tolerance of 1 mil on diameters. It took several steps as I got close because of thermal variations. And if you let the teflon get too hot, it (i) warps and (ii) makes nasty carcinogenic vapors, so I had to be slow and patient with it, when I wasn't feeling like either. Polypropylene and polyethylene are fun to machine. Acrylic is okay too, as long as you go slow. The nastiest in the plastics category (composites actually), in my tiny experience, is fiberglass - not that it's hard to machine really, but it just wears down your tools real fast.We also machine a large variety of machinable plastics. They are a clear thrill because the size changes substantially with temperature.
Are you at liberty to say what material is used ? There are now dozens of specialized seawater resistant steels and other alloys. I was surprised to find that titanium is excellent (but expensive, of course) for marine applications. And although titanium isn't terrible machining (or welding) friendly, it isn't too bad either.Right now I am making parts that go in the shore power connection of submarines to seal the electrics from seawater when the sub is submerged.
Right now, I'm part of a pure physics lab - we do not produce any technology (though we build a lot of stuff for our experiments); we do research in fundamental quantum mechanical phenomena in 2-dimensional systems in the solid state.In what technologies does you lab specialize?About 5 years ago, I spent a fair bit of over a year, synthesizing and characterizing high strength permanent magnets, very much like the one you had to drill.
0 -
1001 = "One thousand and one" OR "One thousand one" - either is acceptable. No comma before the "and".
0 -
Thanks for the welcome, Phi.
Does my mass increase with my post-count ?
0 -
I haven't seen the movie but have heard terrible things about it from physicists who have.
http://rogerebert.suntimes.com/apps/pbcs.dll/article?AID=/20040919/ANSWERMAN/40917006/1023
http://rogerebert.suntimes.com/apps/pbcs.dll/article?AID=/20041003/ANSWERMAN/410030301
0 -
Thanks YT !

I just noticed that there's a separate thread meant specifically for introductions, which is where I should have introduced myself. I apologize for the omission.
0 -
I see it now. Thanks for the link anyway.
Neo's solution is correct.
0 -
What do you mean by "favored" ? At equilibrium, nothing is favored, no matter what the value of K. Also, for most reactions, K is not dimensionless; so changing the units of measurement will change the value of K. So the statement about K being greater than or less than 1 must be made with care. What one can say is the following :with the equilibrium constant, when K<1 the reactants are favoured, when K>1 the products are favoured but when K=1...what happens?In commonly used units (mol/L and powers thereof), if K >> 1, then (the reaction goes nearly to completion before reaching equilibrium, or) the equilibrium concentrations of at least one of the reactants is small compared to their initial concentrations AND when K << 1, the change in concentrations of all the reactants is small compared to the initial/equilibrium concentrations.
0 -
It's correct.i get 2.91x10-3 , but not too sure, didn't have time to check working properlyLifeisogood : How will you tackle this problem ?
There are two tools at your disposal :
(i) The ability to write and balance the required equation and infer stoichiometric proportions from it;
(ii) the definition for Kc
Write down what these two tools give you, and if you're still stuck, we'll help you out.
0 -
neo, I can not see the attached picture (?)
As always, start from the definitions.Ka for HF is 6.8x10-4. Calculate the pH of a 0.35 M solution of HF?i dunno how to do it

What is the definition for (or formula describing) Ka of an acid ? Now apply it to the case of HF. To find relations between the quantities in your equation, write down the equilibrium reaction for ionization of HF in aqueous solution.
And what about pH ? How is it defined ? What do you need to know to find its value ? Is this quantity found in the above equation ?
0 -
No' date=' it is not. The last equation by jdurg is the correct equation for this reaction. Whether it is what your teacher wants is likely beyond the ability of anyone here to determine, but it is correct.????I think is wrong.
The above is NOT a balanced equation, and hence can not be real. Did you even count to see if there are equal numbers of F-atoms on both sides ? Someone is giving you bad information. I suggest you stop getting any more from the same source.2ScF3 + 2Ca ---> 2Sc + 2CaF2The above is the real reaction equation.
0 -
thanks Jdurg' date=' but what do you mean that elemental calcium has a charge 0??
Isn't it 2+?? for pure calcium?? [/quote']
Any stable element/compound must be charge neutral. So, for instance, CaO will have total charge 0, as will Ca (metal). In the first compound, the Ca-ion has a 2+ charge on it and the O ion has a 2- charge, making the whole thing have 0 charge. In the case of elemental Ca, it must have 0 charge to be stable by itself.
Undoubtedly, your textbook will have this covered. Give it a look-see.
Transtion metals are those metals (all the elements, really) that form the d-block of the periodic table. Everything from Sc (top left) to Uub (yet to be named - atomic number 112) in the middle section of the periodic table, is a transition metal. They make up the pink blocks at http://www.webelements.com. Transition metals are characterized by having partially filled d-subshells (exceptions : Zn, Cd, Hg, Uub - which are sometimes considered transition metals and sometimes not). Other (non-transition) metals incluse the alkali and alkaline earth (Groups I,II; blue blocks - known as s-block elements), the Lanthanides and Actinides (green blocks - known as f-block elements) and the following p-block elements :Al, Ga, In, Sn, Tl, Pb, Bi. Their neighbors to the right are classified as semi-metals and those to their right are non-metals.Secondly, Do you guys know what is the difference between Metal and Transition Metal??
Transition metals are themselves metals, so that sentence woudn't be right. However, if you said that transition metals are more stable than other metals, that would be often correct.The only thing I know is Transition Metal is more stable than Metal, but any more significant difference??Another important characteristic of transition metals is that they form colored salts. The reason for the emission/reflection spectra of these salts being in the visible range (thats what it means when something is colored) is that these are the frequencies/energies associated with electronic transitions between two d-subshell energy levels (refered to as [imath]e_g~and ~t_{2g} [/imath] by spectroscopists) that form in a crystal. It is because of these transitions that these elements have this name.
0 -
Guess that hinges on the word 'explain'. If you choose to use a compact Calabi-Yau space to describe some physics (as is done in string theories), then you know the characteristics of this space, and it is well-defined. If the physics that comes out of using "it" is verified (eperimentally), then there is no more "explaining" to be done.Explained how, with one of thousands of potential Calabi-Yau spaces?By saying "they have been explained" I was merely implying that the space in which the physics "works" is well-defined and its characteristics are well-explained. And the usefulness of using such a space is known too.
0 -
XeF6 is closer but still too heavy...
0 -
What is the point in giving away solutions for free ?
lifeisogood : Do you understand what a Std Reduction Potential applies to, and what is the difference between [imath]\Delta E^0 [/imath] and [imath]\Delta E[/imath] ? The Nernst Equation tells you how to get one from the other.
0 -
Look into the Einstein-de Haas Effect and see if that looks interesting/feasible. It's a little tricky to get the experimental sensitivity, but it can be done at the high-school level (with a little patience and a lot of care and attention to detail). Besides, who can resist demonstrating a real quantum phenomenon ?
0 -
Thanks JonB and coquina.
Ah, so you're in Newport News or thereabouts ?I'll join in the welcome. I own a machine shop, & among other things, we make parts (usually one-offs) in support of experiments at Jefferson Lab's accelerator, and often have to machine unusual materials.
"Expertise" ? You flatter me ! But your good looks and sweet words will not win me overI'll probably be calling on your expertise from time to time. . Besides, I really haven't very much experience machining any unusual materials. And you know how far theoretical knowledge goes when it comes to machining !
. Besides, I really haven't very much experience machining any unusual materials. And you know how far theoretical knowledge goes when it comes to machining !  You don't know it until you do it, and you don't believe anyone who tells you it won't work, unless they've tried it first (and even then, you're skeptical).
You don't know it until you do it, and you don't believe anyone who tells you it won't work, unless they've tried it first (and even then, you're skeptical). 
What kind of unusual materials do you machine for the folks at JLAB ? I would imagine most of their metal is 304 Stainless. Well, actually, when I think about it, there's a few others that come to mind - beryllium windows for x-rays, possibly cadmium or zirconium rods, molybdenum targets, etc.
0 -
I hope you're not sniffing too much of that one either !Bad: ... bromine... 0
0


intx^x
in Mathematics
Posted
[sidetrack]If Dave's a 'boing', can I be a 'bling' ? [/sidetrack]