Everything posted by observer1
-
sodium bicarbonate decomposition temperature
NaHCO₃ ---> (heat) Na2CO3 + H2O + CO2 How much heat is required to turn one mole of NaHCO3 to Na2CO3 + H2O + CO2
-
calcium carbonate decomposition temperature
CaCO3 --->(heat) CaO + CO2 How much heat is needed for turning 2 grams of CaCO3 into CaO and CO2
-
IUPAC name
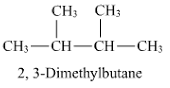
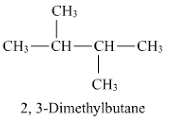
the chemical name of 2,3-dimethyl butane has structure with a ch3 up and down. is it possible for both to be up? what is the name given to that? do the have same/similar properites or different?
-
copper sulphate and water
why is it when water is added to copper sulphate, the hydrogen does not displace the copper and form sulfuric acid? hydrogen is more reactive than copper in the reactivity series H2O + CuSO4 ---> H2SO4+ Cu?
-
cell colour
so it is possible but non-existent
-
cell colour
but in a flat non-rocky and non-sandy area, they are weak. if there are there receptors and color changes, they can use them in any terrain and type of climate
-
cell colour
ok so chameleons change the color by changing the refraction light to form desires color. So theocratically, if there are some kind of nerve that detect the color of light( possibly cones) and send the signal to the other side and the cells recreate the color input to reflect the same, it could be a very camouflaged animal an possibly invisible.
-
Nuclear atom
so it is possible but rare to find it
-
cellulose to glucose
i mean it is not cheap or like cant be made in the house
-
cellulose to glucose
what?
-
cellulose to glucose
how do it turn cellulose to glucose in the most cheapest way possible? here cellulose is paper (no ink)
-
Nuclear atom
but since i have "observed" you, you have to decide which one
-
Nuclear atom
so are you saying that sarcastically or actually?
-
Nuclear atom
so what determines the energy released during a nuclear fission?
-
Nuclear atom
so the more unstable the more energy it releases?
-
Nuclear atom
ok so basically, a standard nuclear fission says if one neutron if fired onto an unstable atom heavy atom it splits into two lighter atoms and 2 neutrons and continues the chain reaction. I am asking if there an atom that is heavy and unstable and when hit by a neutron releases only one neutron or even close to one on average.
-
Nuclear atom
so is there a particle that on average gives one or near one neutron when split?
-
Nuclear atom
Is there a particle which, when is hit with a neutron decays into another atom and releases only ONE neutron?
-
with and without chlorophyll
how do we find a suitable catalyst? like what are the processes to find and test them?
-
with and without chlorophyll
is there a alternative catalyst for chlorophyll
-
with and without chlorophyll
no free oxygen and food then
-
with and without chlorophyll
In photosynthesis, chlorophyll is the catalyst which helps in forming of glucose and oxygen in plants. Now, since a catalyst DECREASES the activation temperature, the plant can use just sunlight as the energy input. Now, how much temperature is needed to make glucose without chlorophyll. Like in a test tube. Photosynthesis Formula:- 6CO2 + 6H2O ->(chlorophyll, sunlight) C6H12O6 + 6O2
- N to N2
-
N to N2
is there a way to close the topic or should i just leave it @exchemist
-
N to N2
oh well....... there goes my idea of using the energy released when N becomes N2 for fuel.