-
Posts
48 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by spamonkey8
-
I dug a nice big toroidal power supply from a broken 400w RMS speaker set and I'm trying to get it to work to supply low (relatively) voltage and a decent amount of current. It has three leads on one side and two on the opposite. Red/white and yellow/orange/black in case it matters. The resistances I read are as follows: Red - White 2.1 Yellow - Orange 0.9 Black - Orange 0.9 Black - Yellow 0.9 (I don't have any way to measure inductors, so this is a rough guess) Now before I tested this out, I hooked the yellow & orange to 110 VAC wall socket and it, of course, flipped the breaker. How can this be used to step down AC voltage if Ohm's law says that the minimum current that would be drawn would be 52 amperes? If pictures help, I can provide them. Thanks!
-

Electrolysis - Electrode Materials, Factors Affecting Rate...
spamonkey8 replied to spamonkey8's topic in Inorganic Chemistry
Depending on certain factors, such as the relation between amperage drawn and electrode size, more or less water vapor is produced. As a side note, anyone who recommended sulfuric acid should be shot. It produces hydrogen sulfide gas when it electrolyzes. Not only is it a pollutant, but it is so because once it hits water, it turns back into sulfuric acid, say on the inside of your lungs. NaHCO3 (baking soda) produces some CO2 I think NaOH and KOH Simply produce a little sodium or potassium deposits on the bottom. However, both are a bit caustic compared to baking soda. So I'd say, baking soda for test runs and once you're sure it won't spring a leak and spray pressurized electrolytic fluid on you, you can switch to caustic lye (NaOH). Just in case anyone still cares. -
Like I mentioned with the cockroaches, I think a mayfly's ecological niche is dependent upon its phisiology to such an extent that deviations that could potentially provide it with a longer life would be impossible to obtain. I know that prehistoric insects were exceedingly large compared to today's specimens, but as far as age goes, the only insects I know of that are capable of living for a substantial length of time would be 18 year cicadas, which is largely spent in incubation. As a general rule, no insect as small as a mayfly could live exorbitant lengths of time. Granted there is evidence opposing the idea, but I think that there are circumstances that take precedence over lifespan. If a mayfly is born that can live for years, it may be so only having sacrificed certain aspects required for its survival. Instead, it simply has a colossal reproductive yield to help make up for it. Again, as Phil pointed out, it's group selection. Whether your kids are "better" than you doesn't matter. If you let them reproduce, you get grandkids that have a 0.25 relationship to you (25% chance of inheriting a particular gene from you), but you say "screw them", be selfish, and reproduce yourself, you make offspring with a 0.5 relationship. As you can see, once again, cheaters prosper. This is all assuming that the grandfathers don't just look at grandma and say "screw her", going for the sexier/more fit younger generation. (I don't mean inbreeding, just consider how many horny old men get a prescription for Viagra and get a trophy wife in her 20's) This works both ways (grandma-grandpa & vice versa)
-
So perhaps the limiting factor of a species' lifespan is a function of how much it must adapt. In the case of crocodiles (great white sharks as well, to an extent), as has already been mentioned, the species as a whole has not needed to adapt greatly. So another point comes to mind: a species that needs to change less would, over time, generally be abe to live longer (with less early deaths due to more fit individuals as any age would be more or less equal), and therefore those individuals able to take advantage of the longer life would be naturally selected, as they would have more time to breed. Bottom line, maybe we die because we have not been given more chance to live. If each generation is easily/likely improved upon by subsequent generations, a longer life's affect on reproduction would be easily offset by the exceeding fitness of adapted young. While the common cockroach has changed little over millenia and yet does not live long (a seeming exception to my theory), I would like to call attention to the general rule that insects and small creatures have shorter lifespans. A cockroach's niche relies on the quick, small, and resilient characteristics only available to a small insect, and this takes precedence over longevity. Thank you all so much for your thoughts, and I'm eager to hear more!
-

Electrolysis - Electrode Materials, Factors Affecting Rate...
spamonkey8 replied to spamonkey8's topic in Inorganic Chemistry
I have one last question for you guys: If the water is heated up to the boiling point, would water vapor be emitted, or would it just perform steam electrolysis? (http://www.google.com/search?hl=en&ie=UTF-8&oe=UTF-8&q=%22Steam+electrolysis%22%3A). -
I've heard a few interesting theories relating to the fundamental reason why all living cells eventually deteriorate, and a new one occurred to me. First, I'll relate the most notable, which is based upon biochemical reasoning (don't worry, it's not hard to understand). Oxygen, being nearly the most electronegative of the elements, has a tendency to 'steal' electrons from other compunds. One theory states that cell aging is derived from the slow breakdown of individual molecules that eventually lead to cell death. A notable byciclist whose name eludes me won the Tour de France a few times and then was forced to retire due to a very rare illness. It was determined that his cardiopulminary system was extraordinarily able to cycle blood and oxygenate it far beyond his peers. His disease was related to the mitochondrion in his cells (the part of the cell that uses oxygen to make the cell's energy, ATP). His mitochondrion were all failing and deteriorating. According to that theory, the increased flow of oxygen accellerated the decay that would normally have occurred. It is interesting to note that in this theory, our very life force is our inescapable undoing. My theory relates to evolution and a species' lifespan. The now cliche phrase 'survival of the fittest' tells us that the species able to adapt the quickest to a changing environment will survive to procreate. To explain my point, imagine the development of a species that can live for hundreds of years. Assuming that the period of time in which the species is able to reproduce is substantial enough to match that of humans (roughly 35 years or half the average lifespan). This species would be able to pass on its genetic material for many hundreds of years. The problem with this hypothetical species is this: If you extend the idea of employment in a business to the existance of a certain evolution of a species, then this species would have a problem with too little turnover. The older 'employees' would be alive and still reproducing. Many generations could have sprung forth while the ancestor of those generations is still reproducing. It would be like great-great-grandfathers and great-great-grandmothers having babies at the same time as their great-great-grandchildren are. Having the older genetic material still reproducing would limit the species' ability to adapt to changes as a whole. Unable to 'flush out' the previous generations which had not had as many chances to mutate into more fit creatures, the species would die out. The only exception to this, I believe, is us humans. We are the only species on earth able to actively affect our environment and change it to suit us, so as long as we can keep from killing ourselves, we may never be forced to evolve. (In the grand scheme of things) In addition to us, species such as trees and Galapagos turtles live for exceptionally long periods of time, but both plants and reptiles have been noted for having a much longer cycle of adaptation. Thus, if evolution is used to explain a species' lifespan, you must consider that it has never been a viable option for an organism to live for an extraordinary long time. Due to this fact, there would be no evolutionary drive to make a species that did not carry some inherent flaw that inevitably diminished its longevity. I'm eager to hear what you think on the matter, both via replies and the poll.
-

Electrolysis - Electrode Materials, Factors Affecting Rate...
spamonkey8 replied to spamonkey8's topic in Inorganic Chemistry
No, I'm not retarded and no it didn't go back in. I was crimping the balloon so that only some of it was exposed when I was trying to light it. I wasn't trying to light it when it popped. I was just giving up. The regulator was closed and a leak at the seam was what blew it up. -
This was about 5 minutes ago. Short story: I had a balloon full of the perfect explosive ratio of Hydrogen and Oxygen and I tried to connect it to a propane torch head and light it. Well, there was a leak in my seal and it went off like a shotgun, singing hair and flinging rubber shards everywhere. Full Version (probably not worth it) http://www.scienceforums.net/forums/showpost.php?p=171261&postcount=34
-

Electrolysis - Electrode Materials, Factors Affecting Rate...
spamonkey8 replied to spamonkey8's topic in Inorganic Chemistry
I just had a near-death experience. My ears are ringing, it sounded like a shotgun going off. So I had the balloon with Hydrogen and Oxygen gas in it in the perfect ratio to combust. Highly explosive. I went out to my garage with this balloon. I attached it to a propane torch head and opened up the regulator. I lit a cigarette lighter and held it up to the business end like I was lighting propane. So pretty much it was a balloon replacing the propane tank. A couple little pops showed me that it was coming out (igniting little pockets of it), and then the fun started. A leak at the connection sprung somehow and the entire balloon blew up in my face. I was leaning over at the time, and it singed off some of the hair on my leg. So remember, kids. Next time you are about to try to light a balloon full of a perfectly stochiometrically balanced explosive (the most explosive known), either get a flashback arrestor or tape the damn seal better. Next time, I'm just putting H2 in it, and in much smaller quantities for damn sure. This warrants a link from the 'stupidest thing you've ever done' thread. -

Electrolysis - Electrode Materials, Factors Affecting Rate...
spamonkey8 replied to spamonkey8's topic in Inorganic Chemistry
Why burning gas fuels is harder than burning liquid fuels: (and yes I realize liquid fuels have to vaporize first but you get the point) Like all highly flammable gasses, any available amount combusts rapidly with a pop/whoosh sound and a flash. This is true with Hydrogen. Any quality propane or butane torch has a regulator and then a very tiny hole that's almost invisible. After that, it allows the gas to mix with air and directs it through a filter that surrounds the bulk of the flow with smaller parts so that the main portion will remain lit. If you have a propane torch on hand, unscrew the end (the very end, past the regulator knob) and look for that tiny hole. If yours is like mine, turn it on and try to light it. You'll find that just because the gas is coming out doesn't mean it will light. Not only is there no uniform mixture with air, but the pressure keeps the flame from burning down to where the gas is coming out. It's like trying to light the assembled torch from 3 inches away. Yes the gas will burn, but it won't stay lit. In short, Hydrogen gas would rather explode than burn slowly, so you have to force it by shaping the flame, controlling its mix with oxygen, and regulating the flow. I'd like to connect the balloon to that propane torch head and try to light it. I'll get back to you on that. I think that by far the two biggest problems will be these: • Optimizing the conditions of the electrolysis enough to produce a decent amount of product. If I can get a mole of H2 every hour or two, I would have plenty for a decent flame. This may mean obtaining an industrial power supply and/or selling my soul. • Getting the flame to burn consistently. Electrodes Update: As a side note, I remembered that the spark plugs in my car are platinum, and I checked to see if I had the old ones laying around, so I have 4 little pieces of platinum to play with, though I'm not sure if it will matter. In those 4 hours of use, an inch-long length of stainless steel wire worked relatively well, only really wearing down to 1/2 its previous size. The wire at the cathode was just fine, so if I can get enough surface area out of platinum for the anode, I'll be able to get this working without spending exorbitant amounts of money and waiting for shipping. -

Electrolysis - Electrode Materials, Factors Affecting Rate...
spamonkey8 replied to spamonkey8's topic in Inorganic Chemistry
It's been up for about 4 hours and it's still going, but slowly because it's mostly adding pressure. The water is very rusty with some sediment at the bottom due to the stainless steel not working as well as it did yesterday. The ice bath cooled it well enough, but the top is getting hot again (I can shake it up: the bottom is still surprisingly cold). My happy birthday balloon is almost finished! -

Electrolysis - Electrode Materials, Factors Affecting Rate...
spamonkey8 replied to spamonkey8's topic in Inorganic Chemistry
Mhm. I explained that in previous posts. The water got hot enough I was worried it would boil, so I stuck it in a mini ice bath. The balloon is still growing, 19 inches last time I checked. No Smoking allowed! -

Electrolysis - Electrode Materials, Factors Affecting Rate...
spamonkey8 replied to spamonkey8's topic in Inorganic Chemistry
What not if I use carbon? Update: The balloon is 17 inches in circumference, the water level raised about 10mL, and it got reeeely hot. -

Electrolysis - Electrode Materials, Factors Affecting Rate...
spamonkey8 replied to spamonkey8's topic in Inorganic Chemistry
So to decrease resistance, I can give the electrodes a larger surface area, place them closer together, and saturate the solution with the electrolyte (some are better than others). I have another question, though. As the process goes on and I get a little rust floating around, how is the resistance affected? Also, does temperature affect the resistance? I have to dig up my old multimeter. Also, the contacts spark when I connect them to the power, so the resistance can't be terribly high. -

Electrolysis - Electrode Materials, Factors Affecting Rate...
spamonkey8 replied to spamonkey8's topic in Inorganic Chemistry
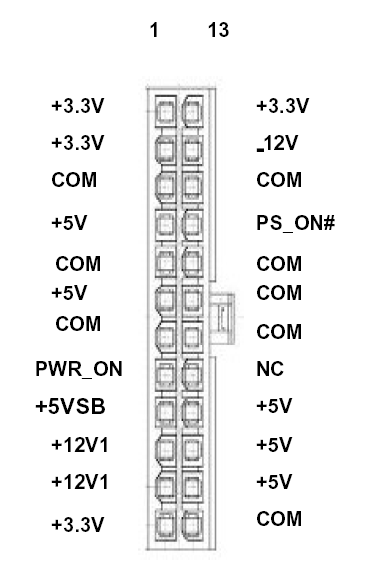
Just a little side note. Even though the PSU I'm using can supply 25A at 5V and 10A at 12V, and 0.8A at -12V and everyone has said that current matters most, using the 12V and -12V leads gives the best results. I attached a pinout for the ATX specification. -

Electrolysis - Electrode Materials, Factors Affecting Rate...
spamonkey8 replied to spamonkey8's topic in Inorganic Chemistry
It looked like carbon deposits, but it was tougher to scratch/rub off than I would guess. At this point, I'd guess the one with the chromate and steel, partially because Occam's razor points me to guess that there isn't a reaction going on that's making a base, producing O2 gas, and blackening only one electrode. I doubt Iron III Oxide as this would be more likely to happen at the anode with Oxygen than with Hydrogen only. I'm starting the balloon a-fillin... This is gonna take soooo long... If anybody wants pictures or has anything to say, let me know -

Electrolysis - Electrode Materials, Factors Affecting Rate...
spamonkey8 replied to spamonkey8's topic in Inorganic Chemistry
Actually the anode has remained remarkably clean over the course of a couple hours with just a slight amber color near the surface. Yes, it rusted slightly, but in that time if it were copper it would have burned away 10 times over. -

Electrolysis - Electrode Materials, Factors Affecting Rate...
spamonkey8 replied to spamonkey8's topic in Inorganic Chemistry
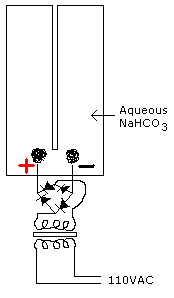
I have a big question! As I said before, I'm getting what looks like carbon buildup on the cathode (negative). But, with only water and sodium bicarbonate (NaHCO3), the only way to get soot like that would be with this: NaHCO3 -> C + NaOH + O2 This is all a guess, but if the above formula is correct, I'm getting: • Soot - If carbon rods will work, then a little carbon buildup can't hurt. • Sodium hydroxide - Just fine as it is a good electrolyte as well assuming no glass or excess CO2. Plus if I add some aluminum, I get Hydrogen gas chemically. I can just make the cathode out of Al. • Oxygen - Uh oh... But wait, the cathode gives me Hydrogen gas and it's an explosive mixture to combine it with Oxygen! -

Electrolysis - Electrode Materials, Factors Affecting Rate...
spamonkey8 replied to spamonkey8's topic in Inorganic Chemistry
That sounded a little funny when I wrote it. Now what explains the less O2 production... Divine intervention always works! The dissolution of the gasses really only impacts Oxygen, as it has a much higher saturation point. I'm about to zip tie/tie with string/tape a baloon onto the mouth of a water bottle and see how it fills. For the electrodes, I'm poking stainless steel wire through near the bottom and using epoxy around the puncture hole. The biggest problem will be keeping the seal of the electrodes from leaking water under the pressure. All this is is really a dry run for looking for potential problems with dealing with the gasses pressurized. I might have to use 2 bottles joined so the gasses are separate and I don't blow my sweet bottom to kingdom come. -
Don't forget that it's half life is 12 years. I don't know exactly how much the average LiIon battery's voltage can drop before it won't power the laptop, but I'd guess half voltage would cause problems. What that boils down to is that a 12 year half-life may only translate into a measly 4 year battery. Wait... Energizer's about to go out of business!
-

Electrolysis - Electrode Materials, Factors Affecting Rate...
spamonkey8 replied to spamonkey8's topic in Inorganic Chemistry
What's the periodic table? Had you there for a second. In the last four and a half hours, I've found out some things that have made my little interest jump a quantum leap. Electrolyte & Electrodes: I found using sodium bicarbonate (simple baking soda) for the electrolyte and stainless steel for the electrodes can allow the process to proceed with no visible residue or deterioriation except that the cathode (negative/ground) gets what looks like carbon buildup (can't hurt at all). This is a major improvement from chlorine gas and chunks of oxidized metal falling off, haha! I figure if I coil the stainless steel and loop it, I can easily get the surface area I need. As far as acids, I don't know if the difference is that noticible, and I'd rather avoid them if possible. If the guys in vegas were giving odds for sodium bicarbonate vs. sulphuric acid, who would I pick? Power: I've been using a computer's power supply (the 12V 10A works better than the 5V 25A to my surprise). I also used a 12VDC 200mA transformer, and though it got really hot in the use, it worked comparably well. I have a couple big-and-honkin heavy transformers from a 400w RMS stereo system that burned out and an old monitor that I can use to get some decent power. One of them is as big as the computer power supply itself, so that should be nice once I figure out what voltage it puts out. Right now I'm running a test setup to make sure this pans out. The water's getting warm, but all that means is I can see less O2 bubbles and everything's happening quicker I assume. I stuck a little sketch on the back end of this post so you can see how I'd split up the gasses. I figure I can make the tubes from PVC pipe and put a valve in the tops and a little pressure gauge if I want. If one tube was 6" the other would have to be about 4.2". I would have a cap to fill it from the oxygen end so that as the H side overflowed with water, I could be sure that there was absolutely no air (read O2) in the H2 side from air left over. It wouldn't really matter in the O2 side. This ended up lots longer than I wanted it...sorry -
(I was about 7) The 9volt battery that kept my alarm clock's time ran out, so I used a stripped extension cord...it started smoking (about 16 yrs.) I used a bottle of lighter fluid to make flaming trails on concrete...in my basement. I barely got away with it after the fire alarms went off. (about 12) After catching crawdads/crayfish in our local creek for fun from time to time, my friend and I decided to eat one. So we lit a fire on the creek bed, boiled water, and cooked one. When I brought back the little steel measuring cup covered in soot and couldn't clean it all off, my mom about killed me. She really would have if she'd found out that we had some gasoline we were playing with too. (14) Soldering connections to a fan in a computer case...with the motherboard still in it. One drop fell and killed it. I returned it and got a brand new one. (16) I somehow managed to format the drive windows was running from while in windows. Not me, but in my senior year in high school, a friend of mine named paul made a dry ice bomb with a nalgene bottle and blew up a toilet at school. He got expelled and we all wore t-shirts that said "Free Paul!" for like 3 weeks. (early teen yrs.) I was taking apart a hard drive and found a little steel wire. It looked strong so I tried to yank it apart and cut two deep slits in my fingers in the attempt. I still have the scars At my birthday party one time I started feeling sick. We had cake and a pool and it went like this: Eat a buttload of cake. Go running around the pool. Barf on the side/in the pool. Feel better and have some more cake while the kindly hispanic maid cleans the chunks up. Repeat! There's probably 50 more I forgot or repressed...
-
I'm looking into electrolysis (using table salt for the electrolyte) and I'm finding that the electrodes don't last very well and they, of course, oxidize very readily. I've tried copper, steel (many kinds available), brass, lead, and even gold plated copper, but none of them stand up. Can anyone think of something that would work better? I have access to a lot of things: tin, lead, steel, iron, copper, brass, alluminum, and more. Also, I'm trying to find out what affects the rate of electrolysis the most, ie, shape of electrode or amount exposed, aspects of the power supply, temperature (warmer water allows more gas to dissolve, O2 is completely dissolved). The one last thing I wanted to know was an estimate of efficiency: watt-hours per mole. So many questions, really just to start an open discussion on improving the production of O2 and H2 gas. So far I've blown up a balloon with hydrogen, breathed some O2 and, of course, blown up a mixture of both. Thanks everyone!