-
Posts
10 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by JC_TheCreation
-
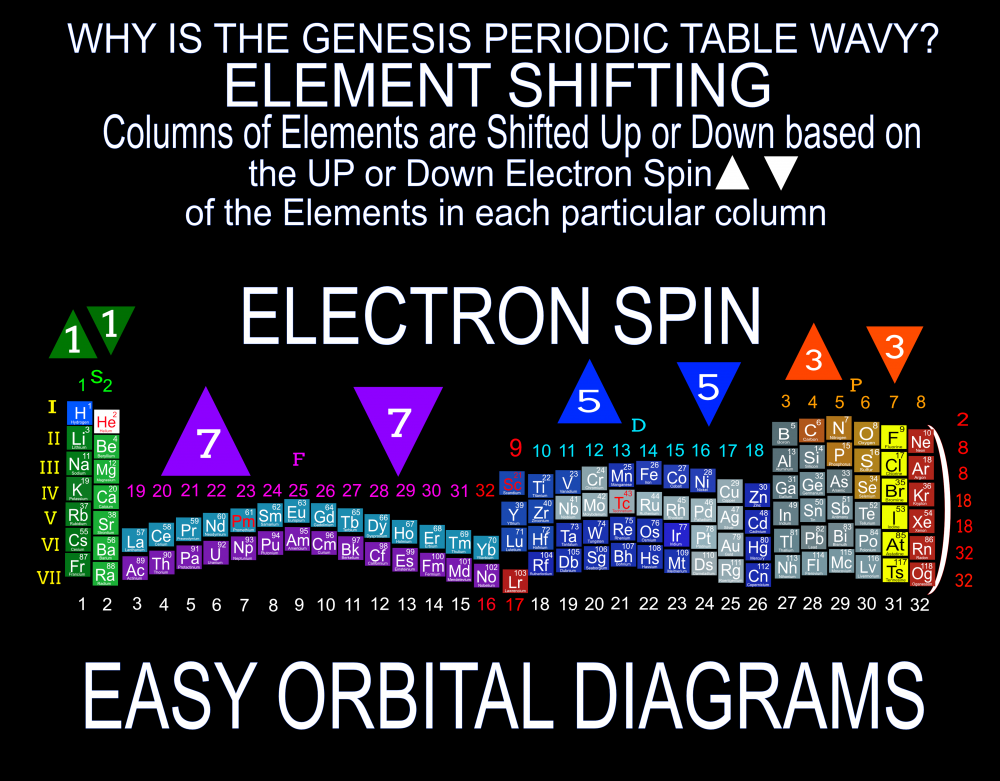
Has anyone ever used the Quantum Fold Periodic Table? Its kind of expensive but I think its pretty awesome that they put the Lanthanides and Actinides in their correct positions and the "Key Folds" are a great way to illustrate the Elements 4th Quantum Number, Electron Spin.
-

IUPAC Periodic Table (1-18) Numbering System
JC_TheCreation replied to JC_TheCreation's topic in Chemistry
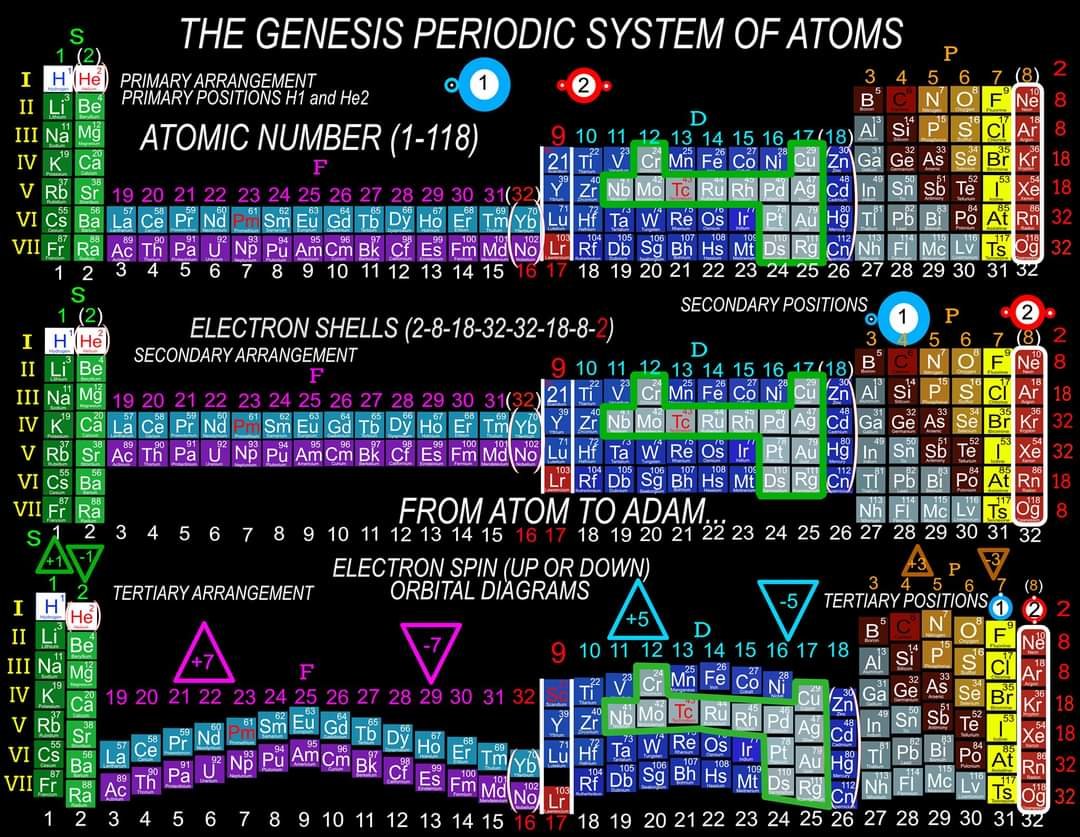
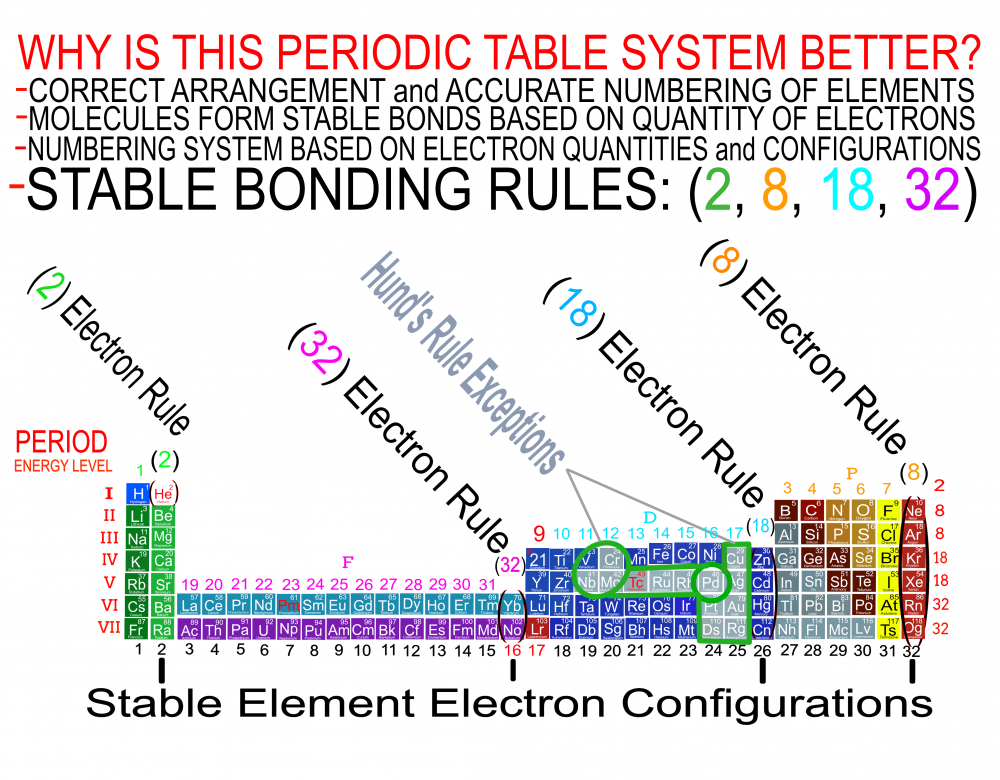
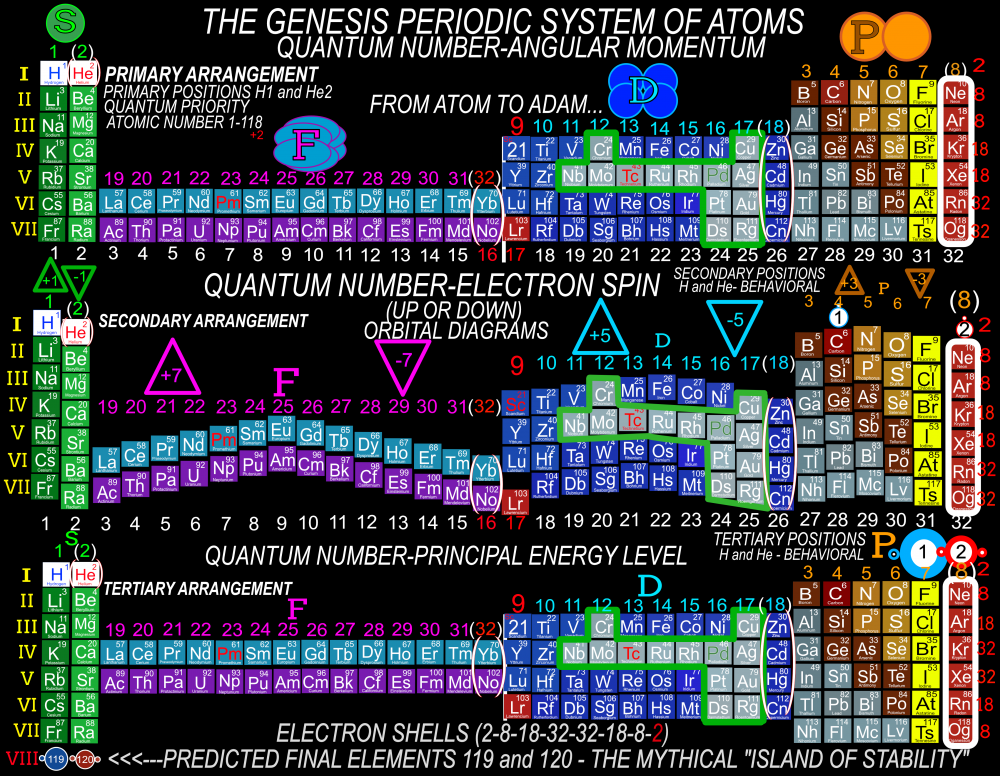
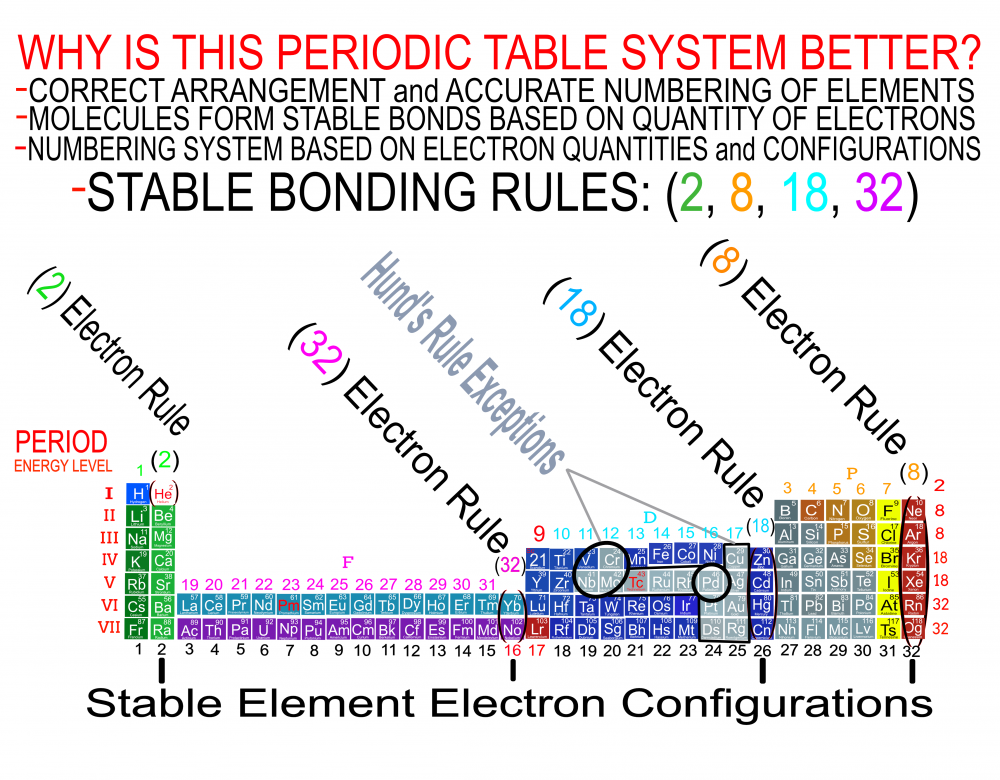
In order for the Periodic System to be a true Universal Language of Science, the primary organizing principle must be the 4 Quantum Numbers and not any Element behavior or properties. Element behavior can change drastically based on conditions such as temperature and pressure whereas the Quantum Numbers are universal. Also, behavior of the Elements is often a function of the Numbers and therefore the Quantum numbers are more fundamental than any Element behavior or property. The devil is in the details and with a System and discovery as important, influential and powerful as the Periodic Table, we must strive to be as accurate as possible when teaching it to the next generation of students. It is fairly simple to prove which Periodic Table System and arrangement is the best by simply counting and listing the number of accurate, useful and complete Element data points that can be extracted by the user at a glance. The Genesis System of Atoms (Primary Arrangement) provides more accurate and complete Element data than any other Periodic Table System currently available. It displays a complete and accurate numbering system that corresponds to the 4 Stable Bonding Rules of Chemistry, 2-8-18-32. Breaking the Periodic Table to "Save Paper Space" is no longer a valid reason as we are shifting more and more away from books to widescreen monitors. Now is the perfect time to make the "Wide", correct arrangement of the Elements the Standard. These arrangements provide measurably more accurate, useful and complete Element data than the Standard. All 4 Quantum Numbers for any Element can be extracted from this system and arrangement without having to reference any separate charts. Secondary Arrangement -
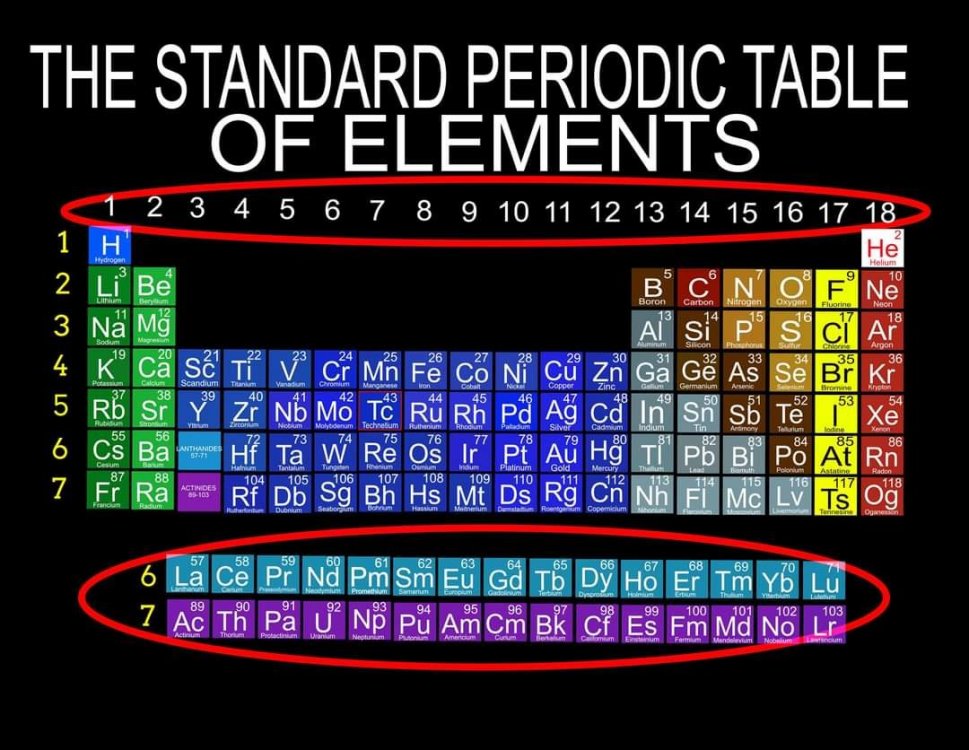
What Element Data does the (1-18) Standard Periodic Table numbering Correspond to? Also, why are the "Rare Earth" Elements always excluded from this numbering system? In my Chemistry Textbooks it just says, "to Save Paper Space" but that doesn't seem like a very scientific reason to remove the Lanthanides and Actinides from their correct positions and exclude them from the numbering as the Standard.
-

Which subshell does the 30th electron belong to?
JC_TheCreation replied to NerdShift's topic in Chemistry
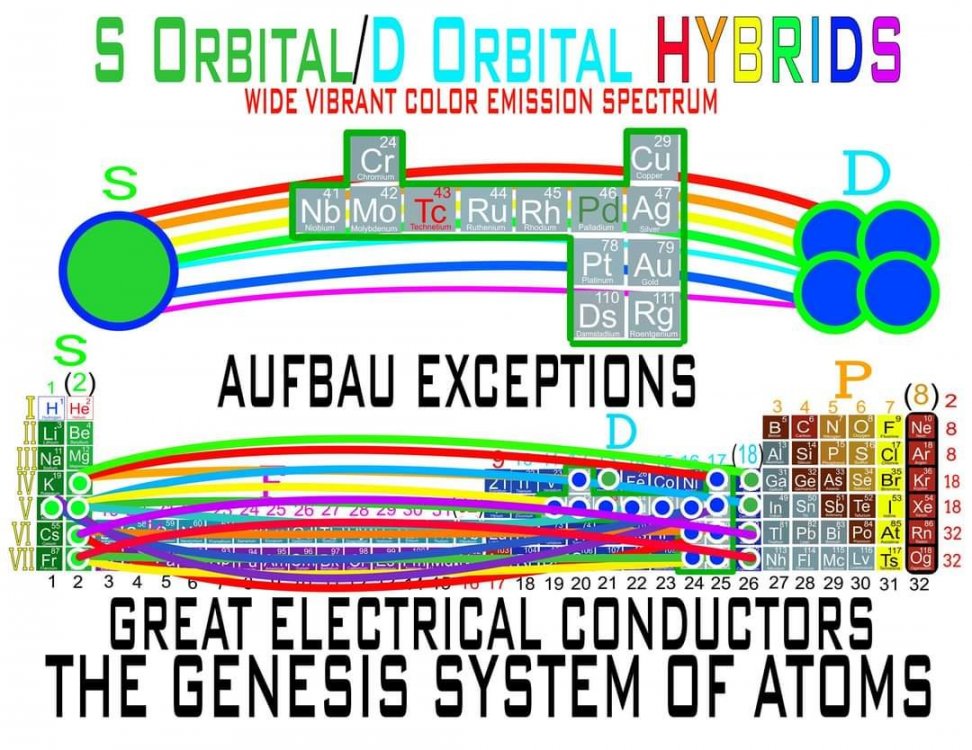
There are several Anomalous Elements in the Transition Metals whose Electrons take from the S Orbital or add to the S Orbital. This is illustrated in the image of the Periodic Table attached, using simple lines to show that these D-Block Elements actually add or take Electrons to/from the S Orbital. Also, 74W Tungsten is NOT one of the Anomalies/Exceptions as it's Electron Configuration is [Xe] 6s2,4f14,5d4 which is exactly what is expected given its position in the Periodic Table. -
Here is the worlds largest online collection of Periodic Tables. https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=1099
-
Quantum Mechanics deals with the behavior of the most fundamental particles and has been one of the most successful theories in human history. The continuing development of a Quantum Periodic Table of Elements is so important to encourage discovery and improve education in all the sciences. I don't know about everyone else, but I have felt like that not enough has been done to create and promote a Periodic Table of Elements System that prioritizes fundamental quantitative data in the arrangement and nomenclature/numbering system. By far the most common and familiar is the Standard Periodic Table of Elements which places many Elements out of Order and severely limits the amount of useful element data that can be extracted from the System. The Lanthanides and Actinides are arbitrarily placed out of order "to save paper space". The (1-18) numbering system does not include the "Rare Earth Elements" and really does not correspond to any Element data. The International Union of Pure and Applied Chemistry has switched back and forth between numbering systems but still have yet to come up with one that's either fully complete or fully accurate. I have still been unable to find a single Periodic Table of Elements anywhere that has a complete and accurate numbering system that corresponds to the simple quantitative element data. So I have always been the type of person where if I don't see something being done that needs to be done, I will just do it myself. I have been working on this for over 8 years and have some of my work published in an editorial Periodical by the Association of Chemistry Teachers http://www.associationofchemistryteachers.org/ISSUE 15 ACT NEWS LETTER.pdf on page 5 and also in their previous issue on the back cover. You can also find my work on Dr. Mark Leech's Worlds Largest Periodic Table Database here https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=1099 and here https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=1124 and I am excited to say that the Genesis Quantum Periodic Table allows the user to extract measurably more accurate and useful Element information than any Periodic Table currently available. No other Periodic Table allows students to easily identify the Electron Spin of any Element for Orbital Diagrams without having to reference a separate chart. In addition, this System also makes it easier to identify and remember periodic trends and anomalies like Technetium, Promethium and Hund's Rule exceptions. Furthermore, having a complete and accurate numbering system makes learning the Stable Bonding Rules of Chemistry intuitive and easy because they are actually built into the Periodic Table System. The Genesis Periodic Table of Elements is a true Quantum Periodic Table of Elements and a Universal Language of Science that is based first and foremost on the fundamental quantitative data of the Elements (Number of Valence Electrons, Number of Core Electrons, Number of Protons and Electron Configurations) with any Element behaviors or properties being secondary components of the system. What this does is it moves Helium over into group 2 based on its 2 Valence Electrons and S Orbital Type. It move the Lanthanides and Actinides back to their correct positions because we barely use books anymore anyways and everyone has widescreen everything so "saving paper space" is an outdated tradition at best and suppression of knowledge at worst. Hopefully it is just something that has been overlooked because this Periodic Table just flat out provides more accurate and complete information and that is what science is supposed to be all about. What is the best and most Red Pill, Mind Blowing way to look at the Atoms and Elements in my opinion, is plotting them 1 by 1 based on the Simple Quantitative Data and the Periodic Law. First, because it is giving you a glimpse into the history of the Universe as Elements were formed smallest to largest, not all at once. When you plot the Elements 1 by 1, say with flash cards, you will find that there are checkpoints such as the one at Element 20-21 where a new type of logic for the numbering System introduces itself. All Elements up to 20 add Valence Electrons and Element 21 is the first Element to add a Core Electron, the numbering system injects a (9) into the middle of the Periodic Table based on the number of Core Electrons in Scandium's D Orbital and the others in that column. If you are using flashcards to plot the Elements, you will discover that you will have to move either 8 or 12 cards to fit Element 21 in its position. Before this, no cards needed to be moved and there were no breaks. The first 20 Elements can be plotted quickly and the logic is very easy to follow. It will likely not be an exercise you will repeat many times, listing all the Elements 1 by 1 by hand, but it is important to see it at least once because it is literally the Elemental History of our Universe and gives us a glimpse into how the Atoms and Periodic Law unfolded. I think its very important that students understand up to Element 20 without the rest of the Periodic Table as this is where the large majority of the Elements in the Universe exist. All DNA, Proteins, Stars and about 99.997% of the Atoms exist within only these first 20 Elements and its a great time to explain how Hydrogen and Helium don't really fit anywhere perfectly and are anomalies of the Periodic Table right away. Helium's traditional position with the noble gases based on its behavior of being an inert gas instead of its quantitative data of having 2 valence Electrons is the reason why the Standard Periodic Table numbering system is always inaccurate. All the Other noble gases have 8 Valence Electrons and closed Electron Shells, yes. But Helium is an S Orbital with 2 Valence Electrons and in Quantum World, Quantity Rules Above Everything, So Helium starts in Group 2 and can slide over to group 8 which will turn to group 0 when it does for the number of Electrons Needed for a stable shell to maintain accuracy in the numbering system. In Fact, Helium's position has been in disputed since its discovery. Because this is a Quantum Periodic Table where we care most about the Fundamental Quantitative Data, Helium starts in Group 2 and has a secondary position in Group 8. Hydrogen also has a Secondary Position over Carbon based on its half filled Electron Shell and Electronegativity and a tertiary position in Group 7 with the Halogens as it only needs 1 Electron for a full shell. When Hydrogen is over the Halogens the numbering system would also need to change to maintain accuracy from 7 to 1 Electron needed for a stable bond. It would take far longer than one post to stress the importance of having a complete and accurate numbering system when it comes to getting the most out of your Periodic Table of Elements. I cant believe its already 2am so that's enough for now. Ill post more of my work if there is any interest. I hope this is making sense and I encourage everyone to test the number of data points in the Genesis Table System vs the Standard Periodic Table. If anyone has any questions about how this Periodic Table arrangement provides more useful and accurate Element data points let me know and I will get back to you as soon as possible. Also any respectful or constructive feedback would be greatly appreciated. Hope you all find this interesting and informative. Blessings Justin Colburn
-
The Periodic Table of Elements is one of the greatest discoveries and developments in the history of science and it remains the most important tool in all of chemistry for making predictions about the behavior of matter. Because the Periodic Table is so powerful, what I am going to say isn't going to be popular or easy for people to accept. It will be far easier to dismiss or delete this post, even though everything that I am going to say is easily verified. The discovery of the Periodic Law by Russian Chemist Dmitri Mendeleev in 1869 launched the academic and scientific communities of the world from studying Alchemy, to Chemistry and Quantum Mechanics virtually over night. Directly coinciding with the 2nd Industrial Revolution, the Periodic Table has become an icon of science and has sit at the cornerstone of the Central Science, Chemistry, for the last 151 years. To the uninitiated, the Periodic Table is nothing more than a nightmare chart that hangs on the walls of high school chemistry laboratories across the globe but to the trained Chemist, it is a Universal language of Science and a window into the underlying order and the structures that build the Universe. A powerful tool that gets its predictive power from the very order that it is built with. Quantitative data such as the number of Valence Electrons and Number of Protons dictate Element location in the Periodic Table as well as determining the Element type, Element properties and Element behavior. Based on where Elements are located within the system, users can extract enormous amounts of useful information at a glance without having to memorize every detail about every Element. The Periodic Table of Elements is literally a Chemistry cheat sheet, but the Standard Periodic Table that you are most familiar with is not the best and most accurate arrangement possible. As important and powerful as the Periodic Table of Elements is, How can that be? Surely that is not true and if it were, someone smarter than me and you would have already figured it out and fixed it, right? Wrong! Unfortunately, it seems more and more common that we have been taught partial truths in standardized curriculum that makes learning sciences like Chemistry harder than it needs to be. Let me break this down for anyone who is still with me. If the Order and Location of the Elements is what gives the Periodic Table system its predictive power, placing Elements in their correct positions is absolutely critical for maximizing the amount of useful information that can be extracted by the user. Its likely you think that the Standard Periodic Table is the best and most accurate arrangement of the Elements because its the most common but if you thought this, you would be dead wrong. The fact is, the Periodic Table you are most familiar with is not the best and most accurate arrangement of the Elements because 30 Elements are routinely placed, "out of order" and excluded from the numbering system for the completely unscientific reason, "to save paper space". The most accurate arrangement of the Elements is reportedly, "too wide to easily fit on a standard size piece of paper", so 30 Elements, often called "Rare Earth Elements" are carved out from the middle, usually with arrows or footnotes glossing over their true positions in the Periodic Table. To make things worse, an inaccurate and incomplete (1-18) numbering system is applied to the rest of the Periodic Table that does not correspond to any actual Element data. I am not making this up, go and have a look for yourself. The International Union of Pure and Applied Chemistry has been responsible for the Periodic Table of Elements Nomenclature for the entire world for the last 101 years and has yet to provide the public with a complete and accurate numbering system for the entire Periodic Table. After 101 years they still cant provide the world with a complete and accurate Periodic Table of Elements System, but I can? How can that be? This is not an opinion or a belief, it is a verifiable fact that I will demonstrate in the following images and video. The current (1-18) numbering system does not include the increasingly important Lanthanide and Actinide Elements which are dogmatically placed out of order below the main Periodic Table out of tradition. In an age of widescreen TVs, widescreen phones, widescreen tablets and widescreen computers, there is no better time than now to make the correct "Wide Periodic Table of Elements" the new Standard. Once all the Elements are placed in their correct positions based on the fundamental quantitative data, more Element information can be extracted and a complete and accurate numbering system can be applied to the entire Periodic Table. The advantages of having a complete and accurate numbering system are many as the Stable Bonding Rules of Chemistry, (the Duet Rule, the Octet Rule, the 18 Electron Rule and the 32 Electron Rule) are reflected in the Periodic Table itself. The devil is in the details and this must be fixed in order to preserve truth! By placing ALL the Elements in their correct positions, it eliminates the need for students to memorize inaccurate nomenclature and simple counting unlocks a beautiful, elegant and more accurate Periodic Table of Elements System. The Genesis Periodic Table is the most accurate, complete and revolutionary improvement to the arrangement of the Elements in over a century. If all the arbitrary and subjective components of the Periodic Table of Elements are removed, what we are left with is a Universal Language of Science that we might use one day to speak to intelligent alien species on the other side of the Cosmos. When all the Elements are in their correct positions, what we are left with is the Genesis Periodic Table. I hope you all find this interesting and informative. God Bless Justin Colburn