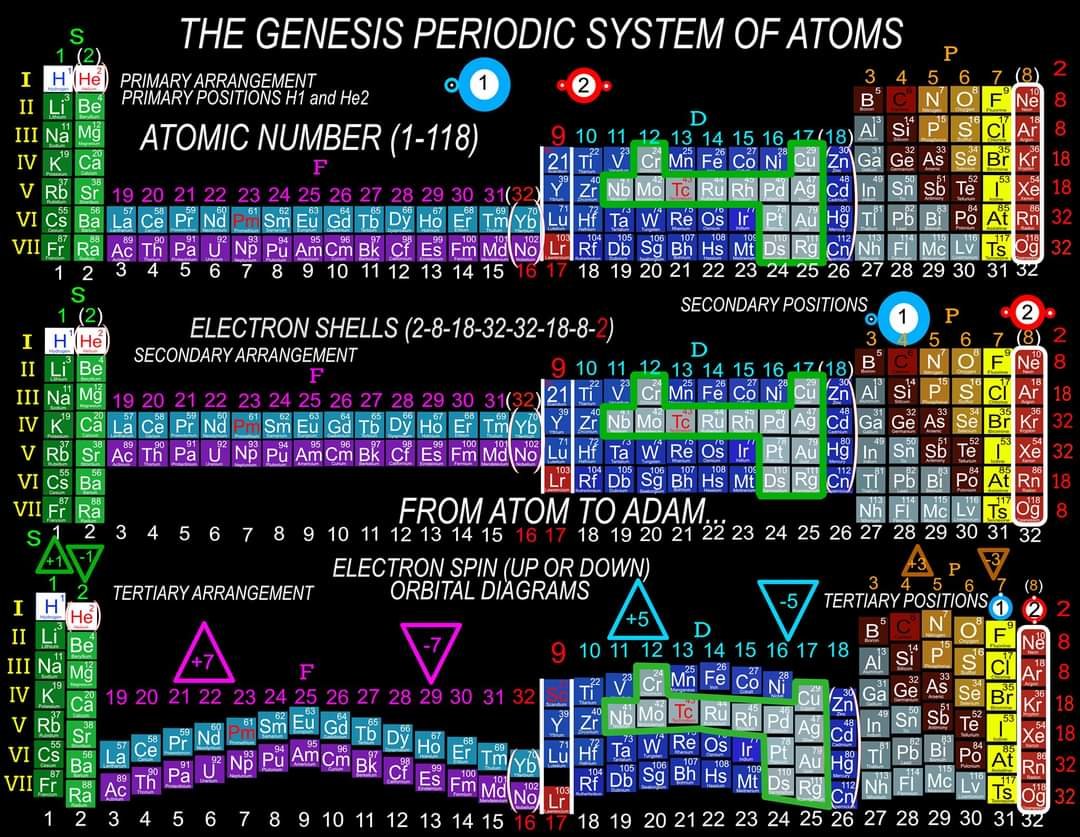
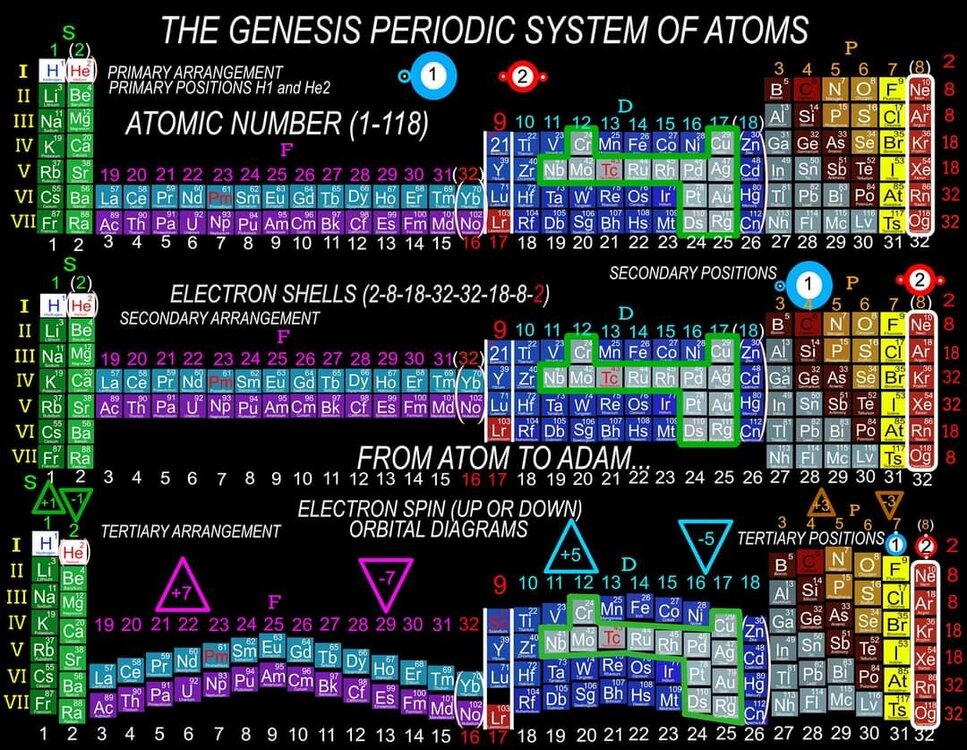
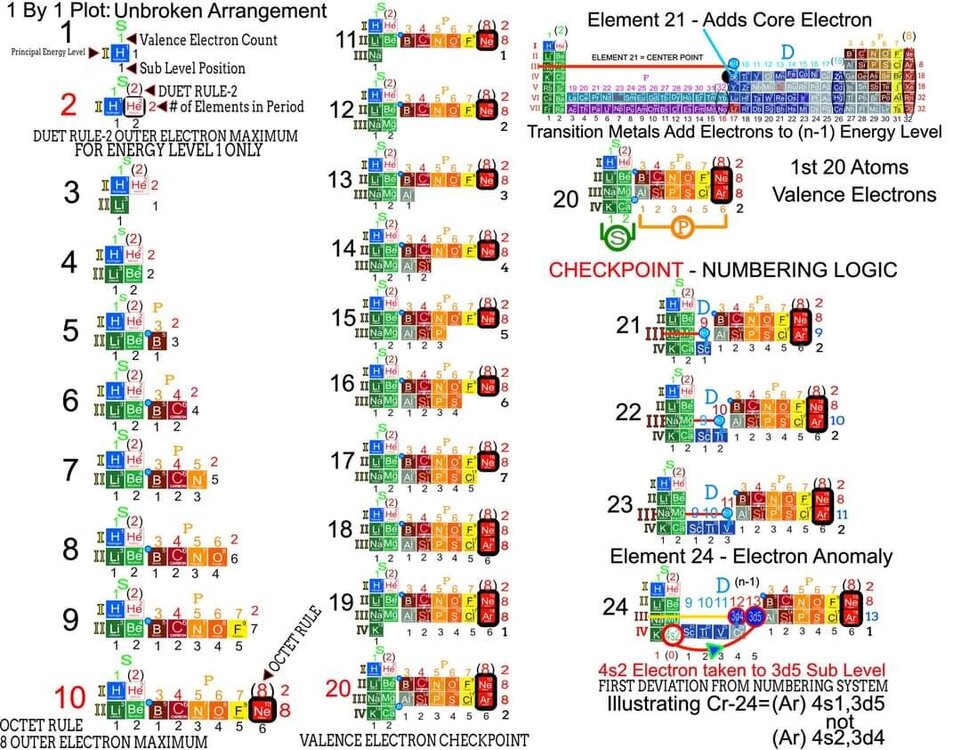
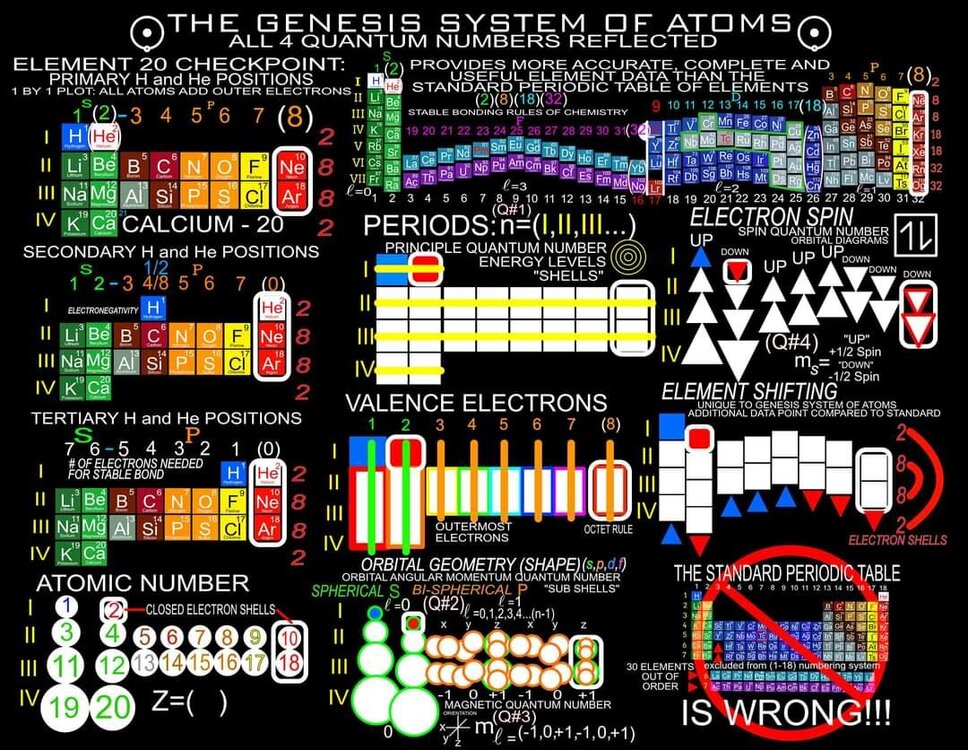
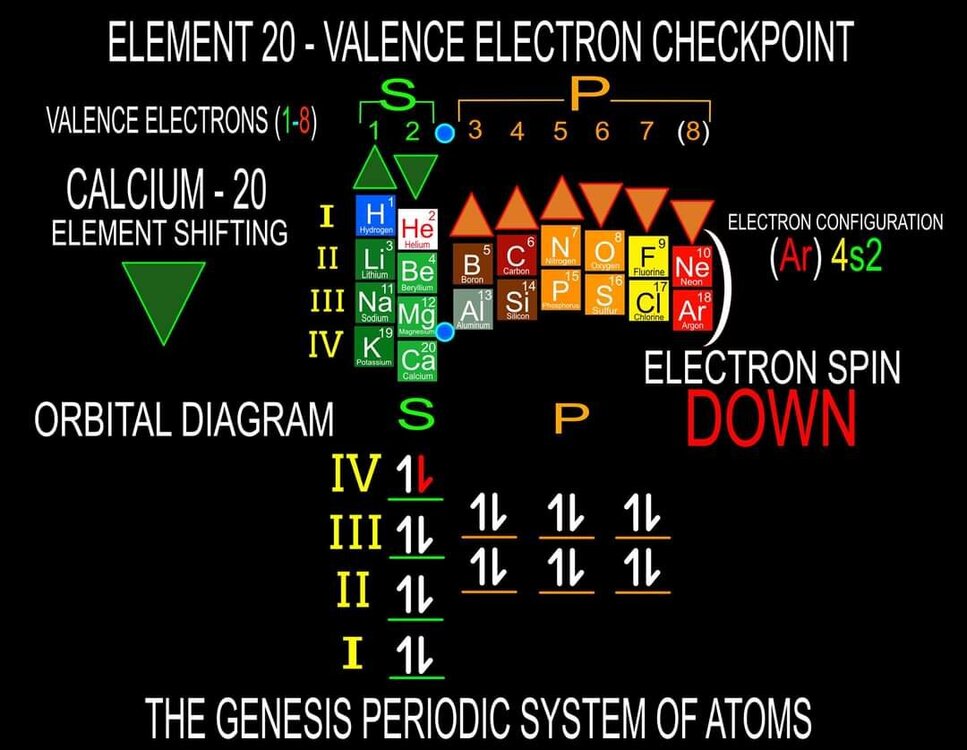
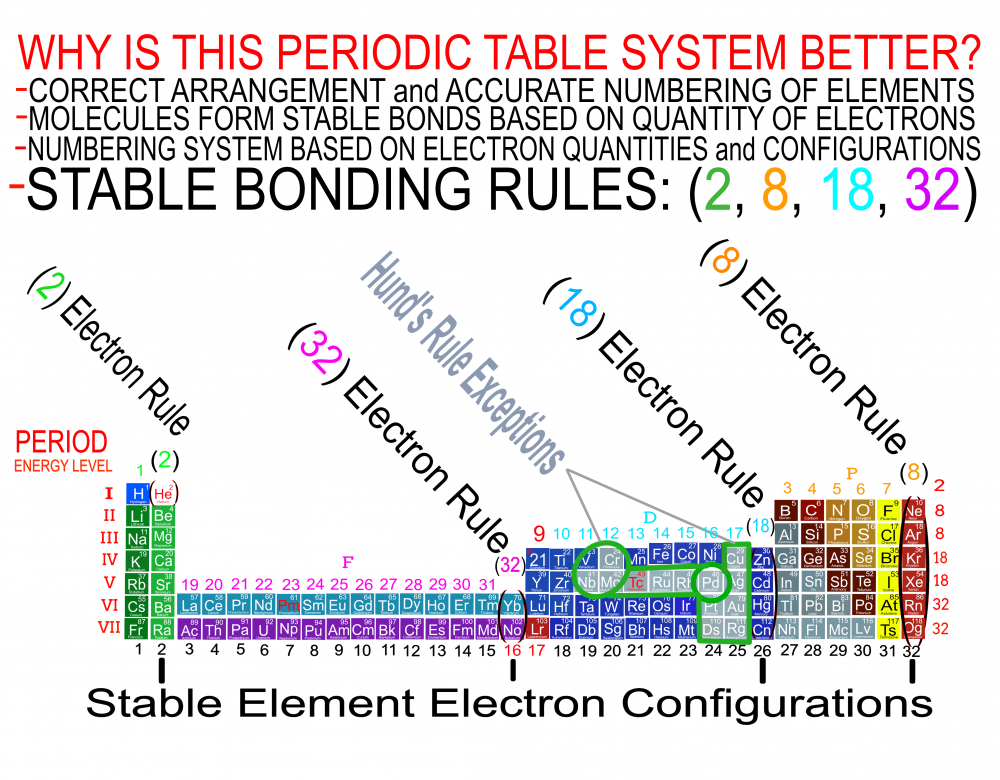
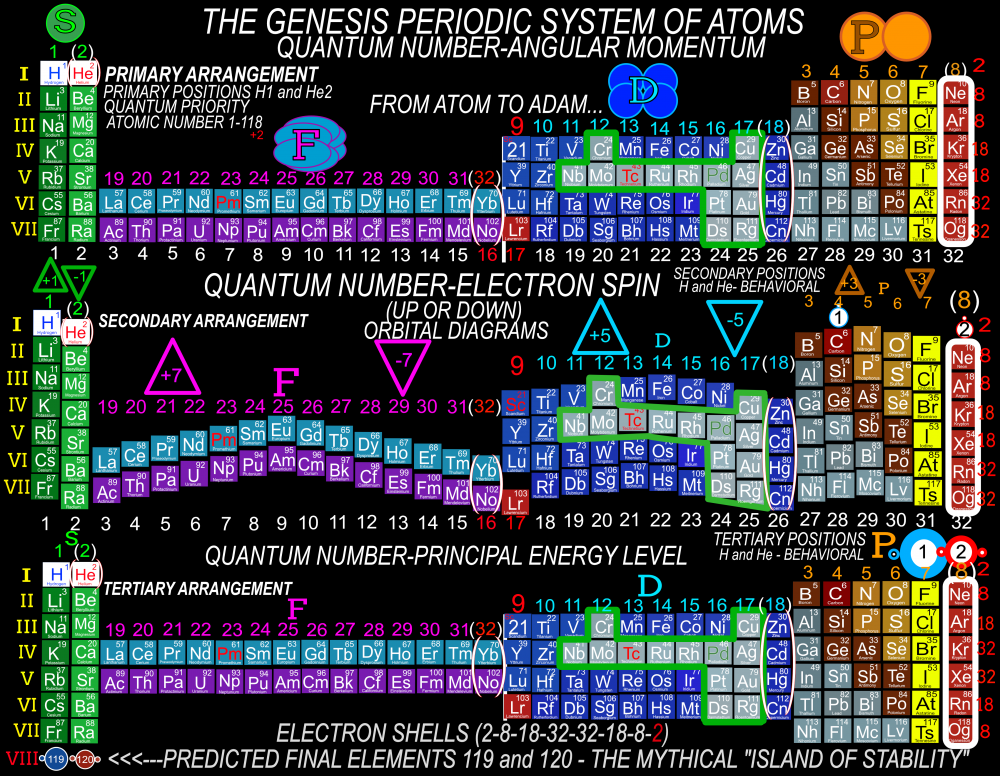
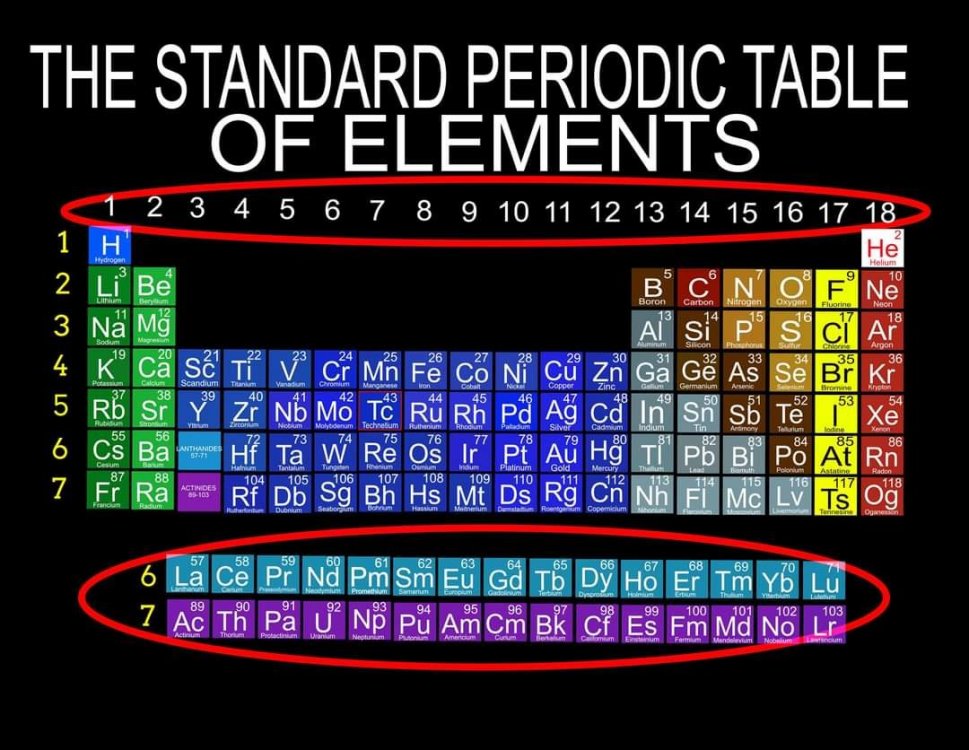
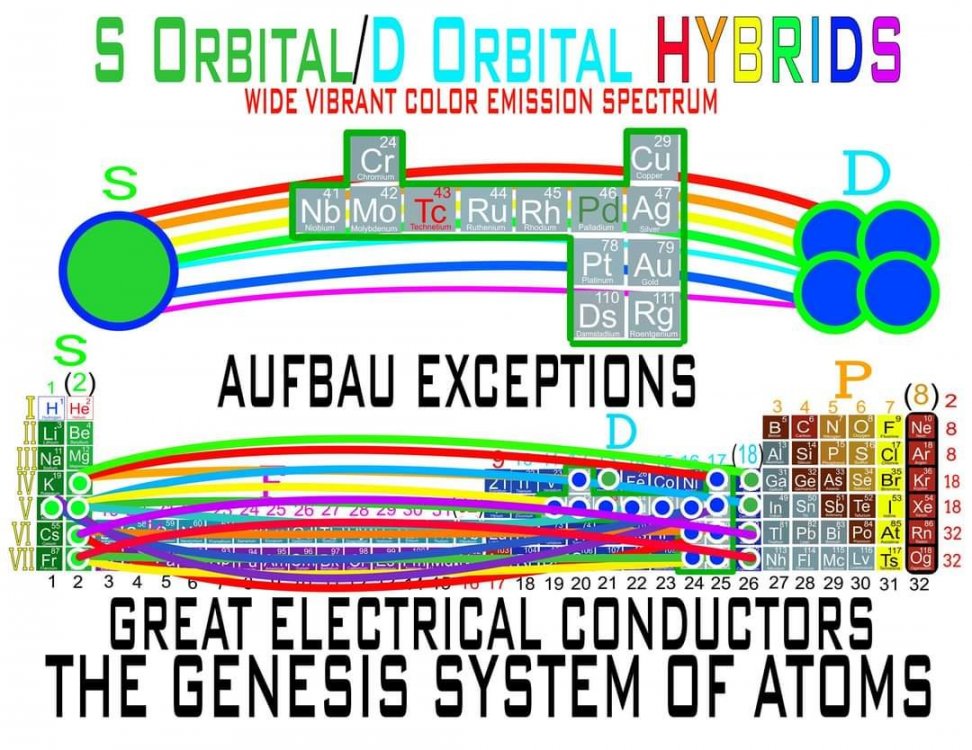
I am proposing a Periodic System of Elements that has the potential to be used as a True Universal Language of Science and one day possibly communicate with an intelligent alien species. I call it The Genesis System of Atoms and it's primary organizing principle is the fundamental quantities of particles within Atoms, the 4 Quantum Numbers and the Periodic Law. The Standard Periodic Table of Elements on the other hand, places Elements based on atomic number, Element properties and an unscientific, outdated tradition, where the Lanthanides and Actinides are placed out of their correct positions and excluded from the Standard (1-18) numbering system. Because of this, and because Element properties can change based on conditions such as temperature and pressure, the Standard Periodic Table is not a great candidate for a Universal Language and would not be a good way to attempt to communicate with an Intelligent Alien Species. The Genesis System of Atoms also emphasizes the importance of plotting the Atoms 1 by 1 in an unbroken arrangement, which provides an Elemental history and Timeline of the creation of the Universe. Because Elements were not all created at once, but rather were created from smallest to largest, plotting the Elements in this manner is a logical and accurate way to present the Periodic System to students and researchers alike. Using the flip book I've created, it brings the Atoms to life while and presents the Genesis System of Atoms to students in smaller, more digestible peices, making it easier to follow, learn and use the most important, influential and powerful discovery and tool in the history of science. The Genesis System of Atoms retains all the Element Data and Periodic Trends from the Standard Periodic Table and only adds additional Element data. It also is the only Periodic System currently available with a complete and accurate numbering system that corresponds to Valence Electrons for S and P Block Elements, (n-1) Core Electrons for D Block Elements and (n-2) Core Electrons for D block Elements. Consequently, the numbering system corresponds to the 4 Stable bonding rules of chemistry, which are 2, 8, 18 and 32. This will allow students to more easily use the Periodic System to make accurate predictions about how Atoms will react and bond with other Atoms. The Genesis System of Atoms, when compared against the Standard Periodic Table, provides more accurate, useful and complete element data to the reader. This is not my opinion or my belief. It is an empirical fact that can be verified with a simple expirement by anyone with the willingness to compare the 2 Systems to the widely accepted element data. The Genesis System of Atoms provides an additional 4 Data Points for EVERY element at a glance. Using the Standard Periodic Table and (1-18) Numbering System, students must memorize and use math tricks, subtracting 10, to find the Number of Valence Electrons for P Block Elements. This may not seem like a big deal to advanced chemistry students but for beginning students it causes nothing but confusing. With the Genesis System of Atoms primary organizing principle being the Quantities of Particles, a complete and accurate numbering system is applied where no memorization or math tricks are required. The Numbering System corresponds directly to the number of Valence Electrons making it easier to extract that data from the System. Additionally, the Genesis System of Atoms numbering system reflects the number of (n-1) Core Electrons for D Block transition metals as D-Block Elements add electrons to the (n-1) Energy Level. The Standard 1-18 numbering system does not correspond to any actual Element Data and excludes the Lanthanides and Actinides completely. The Genesis System of Atoms restores the Lanthanides and Actinides to their proper positions in the arrangement and includes them in the numbering system in a meaningful way. F Block Elements add electrons to the (n-2) energy level and this is easily tracked with the Genesis System of Atoms numbering system. The Genesis System of Atoms also highlights all the Hunds Rule Exceptions that deviate from the expected pattern and numbering system. Instead of shying away from these anomolous Elements, the Genesis System of Atoms tackles it head on and clarifies the hiccups in the electron configurations of Elements like Chromium and Copper that often give chemistry students so many problems. Using the Genesis System of Atoms Numbering System and simple points, lines and arrows, the Hunds Rule Exceptions are able to be graphed and visualized directly within the Framework of The Genesis System. This again, eliminates the need for memorization as this data it is directly integrated into the Genesis Periodic System. Finally, the 4th Quantum Number, Electron Spin is directly integrated into the system using a technique I call, Element Shifting, where columns of elements are shifted slightly either Up or Down based on the Up or Down Spin of the last Electron added to the Atom. This allows students and teachers to identify the Electron Spin of any Element for Orbital Diagrams without having to memorize the information or reference a separate chart. This cannot be done using the Standard Periodic Table. The Periodic System is arguably the most important, influential and powerful comprehensive scientific discovery in the history of the world and it is my position that it should be as fundamental to our education as counting and reading. Everything we can touch, taste, hear, smell and see is made of Atoms so it would stand to reason that the more knowledge a person had about the Elements, the better equipped they will be to live and be successful in the Universe. Unfortunately, the number of people, even those who have graduated from High School or have college degrees, who can read and use the Periodic System to make accurate predictions about matter is staggeringly low. Few know the true power of the knowledge contained within the Periodic System and it is my aim to change that with the Genesis System of Atoms. Thank you for your time if you made it this far and I hope this helps anyone who may be struggling with Chemistry or Quantum Mechanics. I appreciate any feedback and I would be happy to answer any questions. Have a great weekend everyone! Best Regards Justin Colburn