

chenbeier
-
Posts
474 -
Joined
-
Last visited
-
Days Won
1
Content Type
Profiles
Forums
Events
Posts posted by chenbeier
-
-
6 minutes ago, KOLM said:
Wow, Chen, I didnt know. So, even if I were to dry it out some, it would not revert back to CrO3?
Correct, it's difficult to keep it dry after open the container.
0 -
1 hour ago, exchemist said:
Are you referring to chromium trioxide?
Chromium-VI-trioxide CrO3 is called in publics mouth as chromic acid.
If it get wet then it reacts to a sludge
n CrO3 + H2O => H2(CrO3)(n-1)CrO4
With n= 2 for example it will be H2Cr2O7 Dichromic acid.
0 -
For what reason you want to dry it. It's a very poisson and cancerogene stuff. Normally it will be dissolved in water or sulfuric acid as etching compound.
Drying with Phosphorous pentoxide is possible.
0 -
7 hours ago, Dennis1590 said:
My question is. What am I missing here.
You missing it's a weak acid.
The dissociation of carbonic acid is not half H2CO3 half HCO3-. This is only valid if you have a buffer of carbonic acid and (sodium) hydrogencarbonate half half. Then pH is pKa according Hendersson Hasselbalch.
pH = 0.5* (pka-logc) for weak acid.
0 -
But I don't think it's possible to get
CO2 => C + O2 by electrolysis. Also with metal catalysts.
0 -
Only what exchemist already wrote
The CO2 is converted to other compounds, which are conductive.
0 -
1 hour ago, bazzy said:
Hi Everyone
Been A while since i have been on this forum.
I have been looking at a new starship simulator game upcoming and that has got me thinking about oxygen reclamation in a space scenario.
in enclosed spaces like on the ISS they use Lithium Hydroxide to capture and remove co2 while using electrolysis to generate more oxygen. Now I understand that power consumption is an issue with thinking about this, however
If you where to capture and separate the C02 from the air then compress it to a liquid under cryogenic temperatures then as a liquid use electrolysis to break it down to Oxygen and carbon monoxide and oxygen then using Cryogenic Distillation separate the Carbon Monoxide from the Oxygen, then compress the Carbon Monoxide to a liquid and perform the same electrolysis process to separate the Carbon from the oxygen, again using Cryogenic Distillation to separate the remaining Carbon Monoxide from the Oxygen for further processing?
I may be completely missing the mark with the science here but could that work? I do realize this would be an extremely energy intensive process but if energy was in abundance could this process work?
I think he want to electrolyze the liquid carbondioxide itself. But this is not working because it is not conductive.
0 -
-
Because there are some more compounds in the battery like ammonium chloride, the practical voltage is 1,5 V
https://www.azom.com/article.aspx?ArticleID=22363
The voltages can be found in electrical potential tables.
1 -
5 hours ago, Externet said:
Nernst law
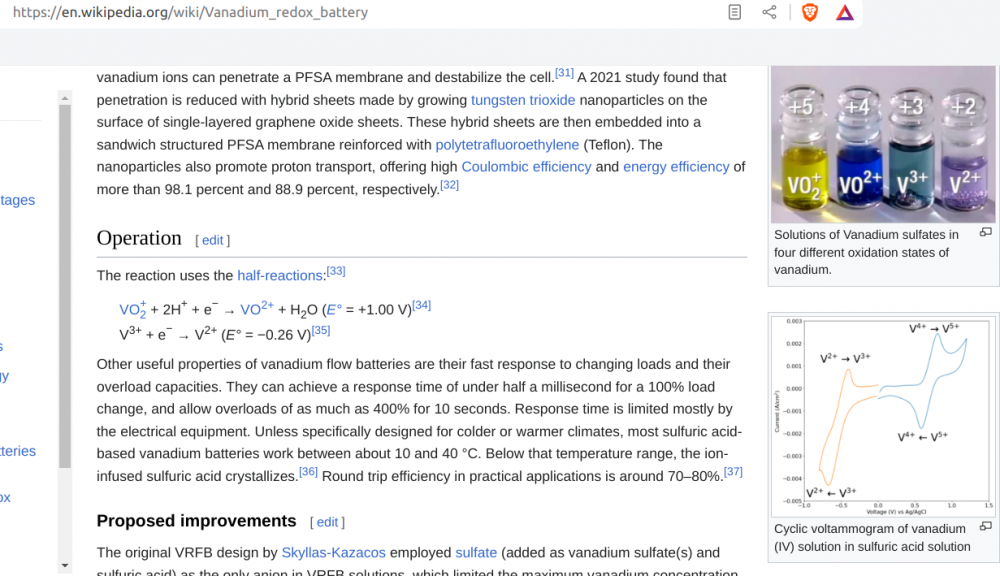
Potontials with positiv voltage minus Potential with more negative voltage gives potential between the two electrodes
Here 1 V -(-0.26 V) = 1.26 V
Zinc -0,763 V carbon-manganeseoxide +0,975 V
0,975V - (-0,763) = 1,738 V
1 -
First what is the volume of your container
Roughly you can calculate you have 3 part H2 and 8 part O2 means together 11 part equal to mol.
Convert Fahrenheit to Kelvin
1058 F = 843,15 K
p = 11 mol*8.314 J/molK* 843,15K/ V m^3
0 -
Of course you will not find it to buy. You have to mix both compounds. It will react immediately.
https://www.researchgate.net/figure/Synthesis-of-urea-glyoxal-resin_fig2_359692764
0 -
That is a poor explanation.
What is your question?
0 -
-
Beside your link I cannot find any other scientific link, where this is mentioned. Its more opposit, lime evaporate the nitrogen as ammonia, it is lost for the plants.
0 -
Quote
carbamide acidifies the soil
Who says that
0 -
The miscibility of liquids and the evaporation rate are different things. If you mix the three solvents they will dissolve directly.
The compound of the highest vapor pressure will evaporate faster as the other ones.
0 -
On 3/5/2024 at 10:23 PM, MigL said:
But pure Ammonia, by itself, is not basic, just a strong reducer ?
Ammonia is a gas at standard conditions, we cannot speak it has an pH, like water steam has no one.
But liquid ammonia has also its autopotolysis like water it has.
2 NH3 => NH4+ + NH2-
2H2O => H3O+ + OH-
So could have a pH scale based on ammonium theoretical.
0 -
Its the opposit
which I reduced an alkane to an alkyne.
You reduce the alkyne to the Alkane.
1 -
Why do you think it will not stop.
If it works it will go through, even you have only two equivalent. Then some reactant will be left.
0 -
If so why should it stop at acetophenon, this could react to 2 Phenyl-2-propanol. But it doesn't happen. If you use instead of the acid the ester then its possible.
0 -
Carbonic acids cannot be treated with Grignard
You can see they also treated CO2 to make carbonic acid. This would be not possible if carbonic acid would under go Grignard.
0 -
Normaly its stable if you convert amines to the amid like acetamid.
https://www.reddit.com/media?url=https%3A%2F%2Fi.redd.it%2F3jehma6dqs2a1.jpg&rdt=60302
0 -
Ok Beside of neutrons, the other make it not radioactive, but it harm in other way as can read here.
https://hps.org/publicinformation/ate/q13412.html
0

Possible to desiccate Chromic acid?
in Inorganic Chemistry
Posted
CrO3, H2CrO4, H2Cr2O7, etc. All are chromic acid.