-
Posts
914 -
Joined
-
Last visited
-
Days Won
3
Content Type
Profiles
Forums
Events
Posts posted by martillo
-
-
For those who could be interested in the new model I have proposed, with no vibrations in the particles (atoms/molecules of a substance), I think I have advanced in the direction of the energies' quantification. Of course the proofs of several (intuitive) assumptions made still remains to be accomplished. I show here just the straightforward quantification already developed only.
I have considered:
H = U + PV is the total energy supplied to the system considered at rest in a Thermal Equilibrium
U = Er = Thermal radiation energy of the photons present in the system
PV = Es + Ek where:
Es = Energy stored in the electromagnetic structure of the substance (electrons' atomic levels and electrons' molecular bonding levels)
Ek = Average Kinetic energy of the particlesThe Equi-partition Principle of the model would apply as:
U = PV = H/2 = 3NKT/2 always
So: H = U + PV = 3NKT always
(K = NKb where Kb is the Boltzmann constant and N the number of the particles in the system)Depending on the nature of the substance of the system:
PV = Es + Ek partitioned someway in Es and Ek
Particular cases:
1) Ideal gases
Es = 0 (negligible)
Ek = 3NKT/2
Er = 3NKT/2
H = Er + Ek = 3NKT
2) Ideal solids
Ek = 0 (static particles)
Es = 3NKT/2
Er = 3NKT/2
H = Er + Es = 3NKT
3) Liquids
Es + Ek = 3NKT/2 partitioned someway (depending on the nature of the substance) in Es and Ek -something between the cases of the ideal gases and ideal solids-
Er = 3NKT/2
H = Er + Es + Ek = 3NKTAs I already said, everything made just intuitively in the calculations must be yet proved valid. I will continue working forward on the subject as far as I could.
0 -
44 minutes ago, Bufofrog said:
Regardless of the efficiency of the turbine, you will have a lot of loss of efficiency by running an entire hydraulic system. It is cheaper and more efficient to just use the engine power directly to turn the wheels.
I don't know precisely with how much efficiently this system could be implemented. Anyway, I think it could have at least its appropriated applications. 2T motors are less efficient than 4T motors nevertheless they are used in some applications like motorbikes, chainsaws, grass cutters, etc.
0 -
I think the main advantage of this hydraulic transmission system stays in its extreme simplicity waiving lot of production costs. I know that also innovative designs in some of it parts would be needed to put it to work but the benefits well pay these things I think.
Just to mention, I'm not interested in any possible patenting rights of the idea. Anyone can make completely free use of it if it where the case. That's why I'm posting it here. If by chance someone could actually be interested to know about the authorship he/she can just send me a personal message requiring the data. I tried to contact some people in the past having no feedback at all. My hope is that it could have useful applications somewhere and not just end lost in the bottom of the drawer.
0 -
3 minutes ago, Bufofrog said:
Looks like the thing would work in principle. However it seems to me that it would be much less efficient than using the pistons to directly rotate a shaft.
It would depend on the efficiency of the hydraulic turbines/motors attached to the wheels, I know.
0 -
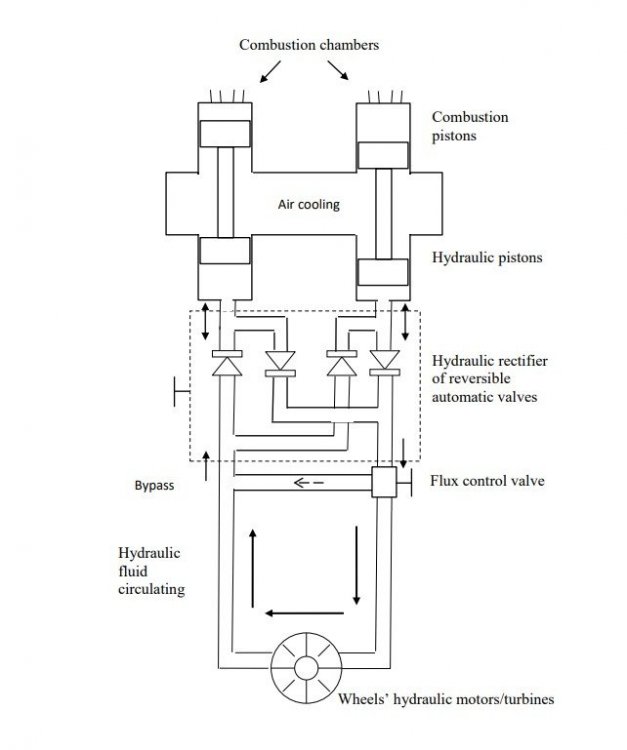
I have developed a very innovative combustion motor with a fully hydraulic transmission system. I'm posting here the diagram with a brief description I made to present it. The aim is to discuss potential problems and/or advantages it could have that I haven't seen on it.
Any comment is welcome.Here is given a schematic description of a combustion motor with a simple but
innovative hydraulic transmission system (based on an adaptation of an electrical device
known as rectifier to hydraulics) which converts an alternating hydraulic flux to a
“continuous” flux with automatic valves. The continuous hydraulic fluid would be
directly transferred to hydraulic motor/turbine(s) to produce shaft rotational power.While on one side the hydraulic fluid descends and the other side ascends following
the alternate sequence of the cycle of admission, compression, combustion and escape,
a continuous flux of hydraulic fluid always moves in the same direction through the
transmission line allowing the motor/turbines rotate the shaft(s).ALTERNATING MOVEMENT STABILITY:
For the stability of the alternating movement of the pistons no flywheel is needed.
Looking at the diagram it can be seen that the system with the hydraulic rectifier works
fine in any configuration of the pistons. The force of combustion on a piston in any
configuration will push it down and the hydraulic fluid will continue circulating always
in the same direction.MOVING IN REVERSE:
The solution is based in the design of reversible valves that could manually reverse
the direction of circulation of the hydraulic fluid. In this case the motor/turbines would
try to rotate in reverse with the hydraulic fluid circulating in the same direction. This
provides also a way to brake the vehicle with the hydraulic motor.AUTOMOBILE APPLICATION FEATURES:
_ In replacement of the conventional transmission and clutch flux control can be
provided with the bypass line.
_ If each wheel has attached its own motor/turbine no differential is needed.FIRST ADVANTAGES:
_ Most of the mechanical parts of the conventional transmission of a combustion
motor system are waived: crankshaft, freewheel, clutch, transmission box and
differential.
_ The overall propulsion system (motor + transmission) would weight much less
with the obvious improvement in efficiency (less fuel consumption for automobile
applications).FINAL NOTES:
_ The motor of two pistons described in the diagram could work well in a 2T
configuration with gasoline, diesel or even other fuels like hydrogen. It can work
without a crankshaft and related mobile parts. In this case a motor starter must be
developed and for motors with sparking an ignition system must also be developed.
_ 4T motors could be developed with at least four pistons. They could be
accomplished with two 2T motors just working in parallel over the same transmission
line (simple connection to a unique hydraulic rectifier) and the four pistons
synchronized with a crankshaft. Similarly, six pistons motors and more could be
developed.
_ Some small hydraulic turbine could move an alternator for battery charge.
0 -
51 minutes ago, swansont said:
A model allows you to do calculations, and accounting for the photons is a critical part of your conjecture. If you can’t quantify the effects, you don’t have a model.
As I have already said I have just begun working on the new model. I just don't have the quantification you are asking at this time. I told you I would do the calculation at the inverse: assuming the energy balance matches in the thermal equilibrium the number of particles emitting at some time could in principle be determined. What I perfectly know is that it is a total waste of time to try to convince you on the model. As I already told you, better for me to invest the time in developing the model further.
0 -
6 minutes ago, swansont said:
Then you don’t have a model. You’re proposing a new description for temperature and you need new physics to make it happen
Yes I do have a model. I just don't have what you need to your calculation. That is not up to me after all. It is you that don't have any proof my model is wrong.
0 -
1 hour ago, swansont said:
If you emit at the rate required for the blackbody radiation the number inside the material is negligible compared to the thermal energy.
Seems to me that a block of solid copper is not a black body.
0 -
1 hour ago, swansont said:
How do you get the number of photons your model requires?
Not possible to know in advance, I think.
0 -
4 hours ago, swansont said:
I didn’t assume they emit at the same time. I assumed they emit at the same rate, because why wouldn’t they? They are identical atoms. And if they don’t emit and absorb at the same average rate, then some area would be emitting more or less than another, which would heat or cool it. But we have thermal equilibrium, so that can’t be the case.
You added the same rate of energy emitted by all atoms to obtain the total radiation energy. That is wrong. The rate and number of emitting atoms to be considered makes the match in the balance of the internal and external radiation energies for the thermal equilibrium take place. Your calculation is wrong.
I could refute all your posted arguments against my proposed model one by one although I know that would lead to an endless discussion. I'm sorry, I'm not able to do this at this time. I cannot continue the discussion. I must concentrate in developing the model further.
0 -
3 hours ago, swansont said:
A model is supposed to make predictions. I took your model and predicted how a solid would behave if it were true. If the established physics I used is wrong, you could point this out, but where?
3 hours ago, swansont said:A model is wrong if it disagrees with observation. And your model disagrees with observation.
As you insist your calculations in your given case is right and for you to not think I didn't consider it properly I will point the main error. As I already said my proposed model must verify energy conservation of course.
You begin calculating the number of atoms in the surface of the block of copper and for a given radiation energy you calculate the number of photons each atom must emit. In the second part you calculate the number of atoms in the entire solid block and calculate the energy of the radiation of all these atoms emitting the same quantity of photons. The error is in you are assuming all those atoms are emitting at the same time or in the same considered interval of time. How you can know in advance how many atoms do really emit at the same time? Nothing guarantees that. Actually the number of atoms emitting must be calculated considering that, at the inverse of your reasoning, both radiations the external one and the internal match to verify a thermal equilibrium state where a perfect verification of the balance in the radiations energies takes place.
I conclude that as for now and having confidence in my reasoning (although aware of capable of making errors sometimes) my proposed model does have good chances.
By the way...
1 hour ago, swansont said:1 hour ago, KJW said:Transparent substances such as gases do emit thermal radiation
Doesn’t really matter; you still can’t reconcile the emitted radiation with the photon gas density you need to account for the thermal energy, since the residence time of the photons is so short.
You seem to think that photons just disappear after some short period of time. You have mentioned this other times too. That is wrong. Photons don't just disappear. Photons don't just vanish. Whatever they are they have momentum and energy which cannot be simply lost or vanish. They can only be converted to other momentum and/or energy and this only happening in the interaction with some other existent particle(s) like the electrons for instance. May be you are confusing the behavior of photons with that of some virtual photons which for instance, like some other virtual particles, can randomly come in and out of existence. Real photons, as bosons they are, don't do that.
0 -
36 minutes ago, swansont said:
Your idea is wildly incompatible with established physics.
So what? For you absolutely nothing could be wrong in established Physics. For me is the established one but this does not prevent it for being wrong in something. As far as I know Physics as any other Science has never been totally infallible and there is a possibility I could be right in something.
You mentioned sometime something like (my words because I don't remember it literally) that Physics' theories are essentially models of what happens in reality and they are considered valid ones while verifying the observational evidence. These are the established theories and I respect that. But what if another model appears also verifying the observational evidence? Then it just must show it can present a better agreement with some observations. Let me say now that I could be finding some such new model and I will work developing it as far as could. It would take time, of course, may be it would need the participation of other ones, of course, and actually there's no guarantee of success at this time, of course. This is not an impediment in my research for me.
What I find at this time is that I must continue some research but not continuing this discussion in this thread now. I mean I could return at some time, I don't know, in this thread or another one, I don't know.
I appreciate your comments very much but unfortunately we are in strong disagreement in some things and I cannot continue discussing now.
My best regards.
0 -
1 hour ago, swansont said:
Your idea doesn’t hold up to scrutiny. It’s not even close.
It's your opinion and your decision. Seems other ones' too. I will continue further with my approach although not here in the forum. Thanks a lot for your comments, they have let me advance a bit while trying to give support to it.
Best regards.
0 -
1 hour ago, swansont said:
But the issue is the energy content. You’ve provided no analysis to show that the thermal energy of the object is from the photons. Just assertion.
My reasoning is that the energy that is supplied to the systems we are analyzing comes in the form of electromagnetic radiation commonly called "heat" which is, in my understanding, entirely composed by photons.
To begin I think in the "empty" cavity with totally reflective walls of a perfect black body is full of photons. It is under this assumption that Planck and Einstein derived their famous "Planck Law" of a black body radiation and "Planck-Einstein formula" E = hf. I then conclude that "heat" is actually composed by photons.
The "heat" transferring between two systems is always through the interchange of photons which can pass through the walls separating them. Photons can pass through atoms and molecules (with scattering dispersion sometimes) carrying energy.
Finally I consider that these photons come into the heated system and cannot just disappear. I can identify that part of them are converted into other forms of energy like the energy levels of electrons in the atoms and also other part is involved in the bonds between the atoms of the molecules. This energies compose the internal energy U in the equation H = U + PV.
My claim is that the energy U is completely ignored in the called Kinetic Theory. In the Kinetic Theory we have U = 0 and H = PV = Kinetic Energy of the particles (translational motion in the ideal gases and vibrational motion in solids). I think that this is not what really happens in reality. As I said the incoming photons do not disappear and part of them is converted to structural forms of energies of the molecules, other part to their kinetic energy and other part just remains as photons in the internal thermal energy.
As I said at some time I'm currently trying to take a mathematical account for how those are energies are distributed in the enthalpy H = U +PV equation. I think I advanced a bit in this direction:
A system (solid, liquid or gas) is heated with an energy ΔH.
Its internal energy U is incremented in ΔU = ΔStr + ΔRad where ΔStr is about the structural energy and ΔRad is about the thermal radiation.
In agreement with Einstein I assume a possible increment Δm in the mass of the particles due to the absorption of energy being Δm = E0/c2 = ΔH/c2 as negligible and so the increment is in PV is Δ(PV) = ΔKE (the increment in the kinetic energy of the particles) .
Then I have: ΔH = ΔU + Δ(PV)= ΔStr + ΔRad + ΔKE.
I think now the Equipartition Principle applies here and so ΔStr = ΔRad = ΔKE = KT and I can have ΔH = 3KT in all cases, solids, liquids and gases.
I just must mention here that for ideal gases we have ΔH = 3KT and not 3KT/2 in the generalized formulation of the Equipartition Theorem applied to ideal gases as it can be seen at Wikipedia page of the theorem: https://en.wikipedia.org/wiki/Equipartition_theorem .
I'm yet still thinking in how the Equipartition Theorem is applied in this approach. I mean thinking in the physical considerations on the ΔStr and ΔRad energies for the Equipartition Theorem to apply.
0 -
3 hours ago, swansont said:
Why should it be? The 2.75 W is dependent on the surface area. Show your work if you disagree.
Thermal equilibrium means the surroundings are at 300K, and 2.75 W is also being absorbed. But that’s at the surface.
When a system is in thermal equilibrium it has the same temperature in any place so the radiated power anywhere is also the same. The Power is the Energy emitted by the area of any radiating volume you can consider inside the system. Particularly a enough small one to identify it as a "place" in the system. The Power divided by the area gives the same value everywhere and it is the temperature in that place.
2 hours ago, sethoflagos said:The internal energy of thermal radiation within a space occupied by a gas is accounted for by the internal energy of the photon gas that co-occupies that same space, Look at the wikipedia page on 'photon gas' for an explanation.
Completely agree with this.
2 hours ago, sethoflagos said:At everyday temperatures, black body photons capable of inducing electron orbital jumps are to all intents and purposes non-existent. The dominant process for generating black body radiation is via the acceleration of charged particles, and as @swansont has pointed out, this can be many orders of magnitude below the internal energy of the matter phase.
There is interchange between the matter phase and proton gas phase, and this can have effects during dynamic changes in thermal equilibrium. Off the top of my head, it's one reason we can never quite achieve the theoretical adiabatic combustion temperature in fuel burning processes. But if you only start getting a glimpse of a phenomenon at >1500 K, there really are no grounds whatsoever for claiming it to be a dominant process at normal, lower temperatures.
Well, I'm currently developing (trying) a different explanation through a different cause for the phenomenon. I'm thinking in the photons continuously exchanged by the particles of a system with their surrounding environment. For instance, in a perfect black body of an empty reflective cavity the particles would be the molecules of the wall of the cavity where it can be considered the reflections the same as an instantaneous absorption and emission of photons.
0 -
1 hour ago, swansont said:
Not all that much. In copper it’s about 0.25 nm. Light would take ~10^-18 sec to traverse the distance
If you had a block of copper, 0.01m on a side, it’s going to radiate 2.75 W at 300K
At 4 atoms per nm, there will be 4 x 10^7 atoms along one dimension. 6 x 1.6 x^10^15 atoms on the surface, so perhaps 10^17 atoms near the surface can radiate outwards. at 300K, the photon energy peak is about 0.1 eV. 2.75 W needs about 2 x 10^20 of these photons per second. So each atom is responsible for about 200 photons/sec, or one every 5 milliseconds. But we know these photons can only live for a time that’s around 10^14 times shorter.
These are estimations, so there might be a factor of 2 here and there that could be off. But you’re ~14 orders of magnitude short
1 cm^3 of copper is about 9g, so the block is somewhere around 10^23 atoms, or 2 x 10^25 photons/s for 10^-18 sec. 2 x 10^7 photons let’s call it 10^8, just to be safe, at 0.1 eV. Which is around 10^-12 J. Compare with the heat capacity of 0.385 J/gK, and we’re at 300K, so we have around a kilojoule of thermal energy in our 9g block.
No, it’s not photons.
I think something is not right in your calculation. You have a piece of copper radiating 2.75 W. If it is in thermal equilibrium the internal thermal radiation is the same as that external radiation of 2.75 W. That is 2.75 Joules per second. It is not about a kilojoule of thermal energy as you say.
0 -
22 minutes ago, joigus said:
Sorry. Here's a lowdown of the vocabulary I've used. Tell me, please, where the problem is:
Degree of freedom
Temperature
Specific heat
internal (rotational/vibrational) vs external (CoM)
cutoff (making some energy --or wavelength-- domain irrelevant; see next)
freezing (as in freezing degrees of freedom by making them very unlikely to store energy under thermal-equilibrium conditions).
Thanks. More clear now. You are talking about the vibrations approach.
0 -
26 minutes ago, swansont said:
If the inside of a solid material the system is at the same temperature, there is no net radiation. Any photon emitted is absorbed, and since c is a big number, the time between these events will be small
For any material system, solid, liquid or gas an average temperature is maintained by the atoms/molecules which stay interchanging only some quantity of photons with the environment. Others are absorbed by them. Remember that after all the there's a lot of space between them.
26 minutes ago, swansont said:This discussion was originally about a solid, and my posts were in that context, but go ahead and calculate the amount of EM energy. The S-B law gives you the radiated power from a surface. What’s the surface area of the interior of a gas? How do you ger an energy content from it?
The surface to be considered is just a representative little "differential" area inside the system through which a "differential" quantity of photons are passing in two directions.
23 minutes ago, joigus said:Absolutely. Sorry I missed this very good argument for so long. It's only because of what you say that different molecules have different specific heats as a function of temperature. The internal degrees of freedom are totally relevant. This is exactly the reason why different molecular components have different specific heats. What other reason could there be for different gases to display different specific heats if only the CoM DOF were relevant?
Quite a different matter is how quantum mechanics introduces a cutoff for short-length degrees of freedom (independently of how poly-atomic a gas is), and how this played a crucial role in the dawning of quantum mechanics itself. (Birth of the old quantum theory as a mechanism to freeze the short-wavelength DOF.)
I'm sorry but I cannot follow you in this. I don't understand the vocabulary properly and may be I'm not capable to put the subject in your terms.
0 -
6 hours ago, KJW said:
I'm not the one who started the discussion on assigning temperature to a single particle. Oddly enough, @martillo rejected my suggestion in favour of something that is not going to work.
3 hours ago, joigus said:Agreed. @martillo's suggestion is not going to work. Your suggestion is, if I understand correctly, a valiant attempt --let's put it that way-- to try and make sense of their hopeless intention to define temperature as an attribute of one molecule or atom.
I think you're right to say that the ergodic theorem is essential to define thermodynamic equilibrium. If most typical physical systems we deal with were not ergodic, I don't think statistical ensembles would work at all within the context of variables such as the partition function, temperature, Helmholtz's free energy, entropy, and such. It would be a disaster. The least I can say is those variables would be as good as useless.
So why bother trying to make sense of something that just doesn't? Just to spite me? Temperature is an ensemble-related parameter. There is no operational definition that would allow us to measure the temperature of a molecule either. There is no theoretical framework that allows us to define it in such a way except by way of the ensemble.
Temperature is an ensemble property. Even more so than entropy is. At least the microscopic entropy of a molecule can be defined as the volume of phase space for that molecule, which is always the same. Not so for temperature.
Fine, if you can't manage my definition of the temperature for a single atom just forget it. My consideration that in the Kinetic Theory of Gases the internal thermal radiation is not taken into account still holds.
As I said I'm considering https://en.wikipedia.org/wiki/Black-body_radiation particularly the following:
On 3/17/2024 at 12:57 AM, martillo said:"Black body[edit]
Main article: Black bodyAll normal (baryonic) matter emits electromagnetic radiation when it has a temperature above absolute zero. The radiation represents a conversion of a body's internal energy into electromagnetic energy, and is therefore called thermal radiation. It is a spontaneous process of radiative distribution of entropy.
Conversely, all normal matter absorbs electromagnetic radiation to some degree. An object that absorbs all radiation falling on it, at all wavelengths, is called a black body. When a black body is at a uniform temperature, its emission has a characteristic frequency distribution that depends on the temperature. Its emission is called blackbody radiation."
The thermal radiation is described mathematically by the Stefan-Boltzmann Law: Pot/A = σT4 where Pot is the Potential of the radiated energy by unity of time.
If the volume of gas cannot be considered as a black body it is just to make the appropriated correction in the law: εσT4 .
The thermal radiation is an EM radiation (photons) and has momentum and energy.
The main claim I'm pointing out is that in the volume of gas in a thermal equilibrium not only the atoms/molecules of the gas are present. There are photons in the volume with momentum and energy and they both contribute to the respective momentum and energy of the total system of gas plus photons in the volume everything counting for the total enthalpy of the system: H = U + PV
If the system is externally heated in some amount there is an increment ΔH = ΔU + Δ(PV).
The problem is in how the increment of this ΔH "heat" is distributed to the ΔU and Δ(PV) in the system. I tried a first mathematical account for everything together but I found it rather complicated for me and I'm not trying anymore for now. If I find something appropriated at some time I would return here.
On 3/15/2024 at 12:00 PM, swansont said:You can’t have motion of atoms, having KE, and have a bunch of EM radiation interior to the system, and have the theory work.
kT is directly related to mv^2 (with a constant related to degrees of freedom)
If there’s energy stored as EM radiation, there’s less KE, but that means a lower temperature.
There would be less kinetic energy in the atoms/molecules of gas (with less average velocity) but this would not imply less temperature. It is compensated by an amount of temperature given by the photons according to the Stefan-Boltzmann Law.
0 -
From Enthalpy (https://en.wikipedia.org/wiki/Enthalpy) :
Definition[edit]
The enthalpy H of a thermodynamic system is defined as the sum of its internal energy and the product of its pressure and volume:[1]
- H = U + p V ,
where U is the internal energy, p is pressure, and V is the volume of the system; p V is sometimes referred to as the pressure energy Ɛp .[6]
Physical interpretation[edit]
The U term is the energy of the system, and the p V term can be interpreted as the work that would be required to "make room" for the system if the pressure of the environment remained constant. When a system, for example, n moles of a gas of volume V at pressure p and temperature T, is created or brought to its present state from absolute zero, energy must be supplied equal to its internal energy U plus p V, where p V is the work done in pushing against the ambient (atmospheric) pressure.
The internal thermal radiation I'm considering (in its two models, EM waves or photons) has momentum and energy both contributing to the enthalpy. Its momentum contributes in the second pV term exerting a force and work while its energy contributes in the first U term the internal energy of the system.
0 -
4 hours ago, KJW said:
Are you
Are you aware that the thermal emission spectrum of an external surface (the thermal radiation inside a cavity is black-body) is equal the product of the absorption spectrum and the black-body distribution? That is, good absorbers of a given wavelength are also good emitters of that wavelength. Therefore, by arranging the absorption spectrum of an object to absorb at all wavelengths other than that of the radiation, emission will be maximised, absorption will be minimised, and the temperature will equilibrate to a minimum which becomes the cold sink of a heat engine; and by arranging the absorption spectrum of another object to absorb at only the wavelengths of the radiation, emission will be minimised, absorption will be maximised, and the temperature will equilibrate to a maximum which becomes the hot source of the heat engine.
That is a subject for another thread. You should open a new thread to discuss it.
0 -
5 minutes ago, KJW said:
A long time ago on a different forum, I made the claim that only perfect black-body radiation, both in terms of its relative frequency distribution and its intensity, can have a single temperature attributed to it. Every other distribution of radiation has a pair of temperatures attributable to it. The lower temperature is defined as the temperature achieved by an absorption spectrum that minimises absorption and maximises emission, while the higher temperature is defined as the temperature achieved by an absorption spectrum that maximises absorption and minimises emission. Then one can construct an ideal heat engine based on these two temperatures to extract work from the radiation. One can't do that if the radiation is perfect black-body as this has only a single temperature.
I don't understand how it would be possible. May be something to discuss in other thread.
0 -
58 minutes ago, KJW said:
It occurred to me how one might do that. One could define the temperature of a single particle in thermal equilibrium with its surrounding environment in terms of the kinetic energy distribution over time of the particle. Applying the ergodic principle transforms this to an ensemble distribution for which the notion of temperature naturally applies.
The thermal equilibrium I'm conceiving is something different. Is not related to the kinetic energy of the particle. I consider the particle is capable to absorb and emit EM radiation (photons). All atoms have their characteristic levels of energy in accordance to the levels of its electrons. All atoms have their characteristic spectra. When electrons jump to other levels they absorb or emit photons. In a thermal equilibrium with its environment the particle continuously absorbs and emits photons maintaining an average level of energy. Those photons constitute the EM radiation present in the environment called thermal radiation and it has a temperature associated to it. The temperature is related to the density of the power of the radiation per unity of area according to the Stefan-Boltzmann Law:
P/A = σT4
0 -
On 3/15/2024 at 12:00 PM, swansont said:
There is no “internal EM energy”
Yes there is. It is called thermal radiation. It is the only thing that exist in the interior of a perfect black body and it is the thing it emits.
See: https://en.wikipedia.org/wiki/Black-body_radiation
For instance:
"Black-body radiation is the thermal electromagnetic radiation within, or surrounding, a body in thermodynamic equilibrium with its environment, emitted by a black body (an idealized opaque, non-reflective body). It has a specific, continuous spectrum of wavelengths, inversely related to intensity, that depend only on the body's temperature, which is assumed, for the sake of calculations and theory, to be uniform and constant.[1][2][3][4]
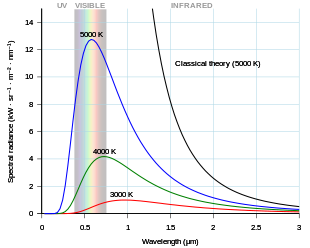 As the temperature of a black body decreases, the emitted thermal radiation decreases in intensity and its maximum moves to longer wavelengths. Shown for comparison is the classical Rayleigh–Jeans law and its ultraviolet catastrophe.
As the temperature of a black body decreases, the emitted thermal radiation decreases in intensity and its maximum moves to longer wavelengths. Shown for comparison is the classical Rayleigh–Jeans law and its ultraviolet catastrophe.
A perfectly insulated enclosure which is in thermal equilibrium internally contains blackbody radiation, and will emit it through a hole made in its wall, provided the hole is small enough to have a negligible effect upon the equilibrium. The thermal radiation spontaneously emitted by many ordinary objects can be approximated as blackbody radiation."
and:
"Black body[edit]
Main article: Black bodyAll normal (baryonic) matter emits electromagnetic radiation when it has a temperature above absolute zero. The radiation represents a conversion of a body's internal energy into electromagnetic energy, and is therefore called thermal radiation. It is a spontaneous process of radiative distribution of entropy.
Conversely, all normal matter absorbs electromagnetic radiation to some degree. An object that absorbs all radiation falling on it, at all wavelengths, is called a black body. When a black body is at a uniform temperature, its emission has a characteristic frequency distribution that depends on the temperature. Its emission is called blackbody radiation."
On 3/15/2024 at 12:00 PM, swansont said:You can’t have motion of atoms, having KE, and have a bunch of EM radiation interior to the system, and have the theory work.
kT is directly related to mv^2 (with a constant related to degrees of freedom)
If there’s energy stored as EM radiation, there’s less KE, but that means a lower temperature.
Yes it works. "kT is directly related to mv^2 (with a constant related to degrees of freedom)" continues to be right. It is just that there's the extra "bunch of EM radiation interior to the system" in the system may be not taken into account.
On 3/15/2024 at 2:47 PM, swansont said:That's the electrostatic interaction, which involves virtual photons. That's typically not referred to as EM radiation, which consists of real photons. The vibrational modes of a solid can be described in terms of phonons (not photons); there are non-radiative ways of changing those states.
If I can explain the thing with real existent particles (the photons) why would I appeal to the mathematical artifice of "virtual particles" (phonons)?
On 3/15/2024 at 4:36 PM, joigus said:On 3/14/2024 at 4:55 AM, martillo said:the temperature of an atom is [...]
There is no such thing.
You mean it is not being considered. I can define it as below.
On 3/15/2024 at 4:36 PM, joigus said:Thermodynamics defines temperature based on thermal equilibrium.
I can define the temperature of an atom (as of that any material object) in thermal equilibrium with its surrounding environment as being the same as that of the environment which can be measured by a thermometer.
0



Hypothesis about temperature (split from Physical mechanism how matter absorbs radiation.)
in Speculations
Posted · Edited by martillo
The answer is yes.
I consider "heat" as the energy flow of photons what is the same as a radiation of photons. As I said other time, there is empty space between atoms and molecules where the photons can pass through. The average velocity of these photons would not be c as there would be scattering dispersion with the atoms and also absorption and re-emission of them in their not linear way. Conductive heat transfer is precisely this phenomenon of photons travelling through dense materials or substances. The phenomenon could be described as the progressive diffusion of photons inside them.
I consider the cosmic background radiation as a radiation of photons particles. I'm considering the particles behavior of heat and light.