-
Posts
7 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by KCM92
-
-
We don't necessarily need it to work in practice, it just needs to be the most efficient theoretical synthesis and an alternative synthesis for bonus points.
With that said, the step converting your dibromide to the diterminal alkenes (step two going from the start material) will not work at all. Yes in theory, you could eliminate form the primary methyl groups to give you the product you want, and maybe this will be the kinetic product...but the more substituted alkenes are more stable (i.e. four groups is more stable than 3 groups which is more stable thn 2 groups which is more stable than 1 group).If I use a bulky base such as t-butoxide the anti-Zaitsev product will form, that's what I intended for elimination of the bromine atoms. I didn't include the reagents on my post.
0 -
-
That helps a lot, thank you very much. I'll talk to my professor about the chirality to make sure.
0 -
Are you thinking of dehydration of an alcohol for the second step? I could do an ozonolyzis on the starting material to both ends, then a Grignard addition with methylmagnesium bromide to get the two hydroxyl groups and appropriate amount of carbons. From there I'm stuck on getting the ring to form.
The problem is written down correctly. I think my professor just wanted to make it slightly more difficult.
Also, am I correct in assuming that I don't have to worry about the stereochemistry because there is only one possible configuration for the final product (the two bonds attached to the oxygen are always going to be on the same plane)?
Thank you for the reply.
0 -
Hi everyone,
I'm Kaleb, an undergrad in chemistry. I've been reading the forums for a while now and finally decided to make an account.
0 -
I have an assignment in my organic class (First semester of organic for chemistry majors) to show how to perform the following synthesis.
We can only use reactions that we have covered in class this semester which include: alkene additions (hydroboration, oxymercuration, etc.), reactions of alcohols (oxidation, converting to tosylates/mesylates, reduction), epoxide formation and breaking reactions, nucleophilic substitution and elimination, metathesis reaction, Grignard reaction, cleavage of glycols. We are graded on using the most efficient method, and get bonus points if we include another method.
I started and am stuck on how to get the starting material to a ring. I'll post what I have so far and I would appreciate any advice on where to go from here.
Step 1 (working backwards):
Either step would work, I assume the peroxyacid one would be more efficient though.
Step 2: (t-butoxide is supposed to be over the reaction arrow)
This is where I am stuck, I'm not sure how to get the starting material into a cyclobutane/ene ring. I have one possible method figured out, but I'm not sure how well it would work.
Any advice/help would be much appreciated. Thanks in advance.
0



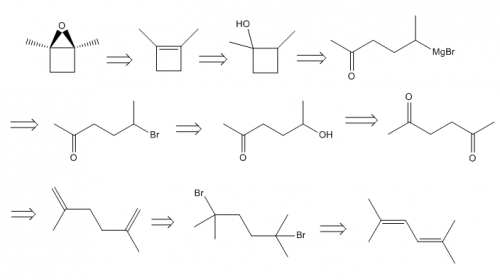
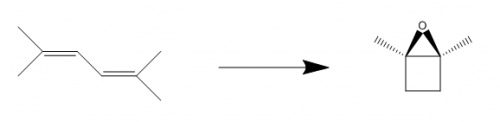
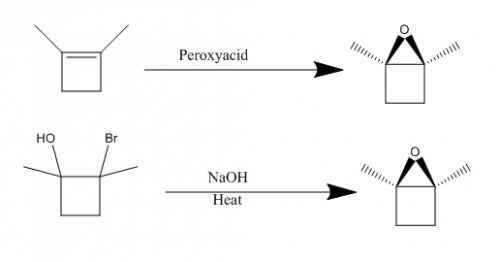
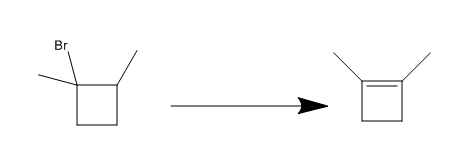
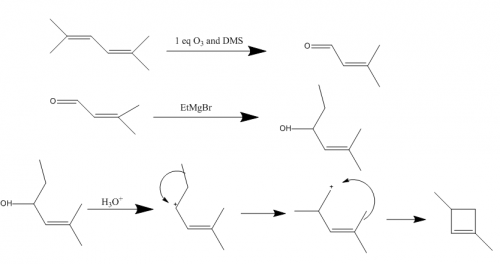
Organic Synthesis Question
in Organic Chemistry
Posted · Edited by KCM92
I have a quick question regarding glucose. If glucose (in pyranose form) is reacted with PBr3 would it be possible to get only the primary alcohol to brominate? My thinking is that since it's much less hindered bromine will add much more quickly to the primary alcohol. Or would it be better to protect all of the alcohol groups, then selectively deprotect the primary alcohol? I'm asking because I need glucose with a bromine where the primary alcohol is located for a step in a synthesis project and am not very familiar with carbohydrate reactions. This is a small part of the overall project that I was unsure about. Also this is for an O-Chem II class just for reference.
Thanks for any advice.
Edit: Here's some more info
The final structure I need to make is below