-
Posts
200 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by esbo
-
-
However if the object and target are not absolutely head on there may be this force
causing the target to me missed.
I am not sure whether the size of the force is proportional to the speed (which would make
things worse)
But I am now.
http://www.regentsprep.org/Regents/physics/phys03/cdeflecte/default.htm
- If the charge on the electron were greater, the force would be greater.
- If the speed of the electron were greater, the. force would be greater
- If the magnetic field strength were greater, the force on the electron would be greater.
- If the mass of the electron were greater, it would have no effect on the force, but the circular path would be larger.
0 -
Moving electric particles tend to create fields which produce force at right angles to the direction of movement
that's how motors work.
So I am just wondering about how that affects things, might not work if they are moving head on though.
0 -
Some pieces fit better than others. Electrons are not observed falling into nuclei and dismissing this fact dismisses all of known modern physics. You are however correct that it is not understood how electrons move about in their orbits and you are very good to question this. I hope you do well in your studies and find answers to the questions you are asking. I'm posting a paper that has been submitted online by a professor from the University of Iceland, it addresses some aspects of the topic. It is a paper that I find very difficult to understand, but I believe I can learn from it at least a little for now. Have fun esbo!
Well I did go to uni but to study electronics, I would rather have done physics maths or chemistry as find the pure subjects much more interesting
but I though electronics would have been better jobs wise, maybe it was initially but not any more really I went into computer programming
in the end, but that went downhill after a while.
I had little interest in electronics and found most of it to be 'rubbish'.
I could possibly do another degree but I don't think that is gonna happen for a number or reason not least cost.
I do sometimes discuss stuff on forums from time to time but it is usually not long before I get my knuckles raped by the mods (lol)
so I don't bother with it so much these days. Science forums seem to be particularly bad for this, free speech seems to have gone out the window
a long time ago.
That paper is a bit too long and mathematical for me to look at at the moment, it is difficult to follow stuff in mathematical symbols unless you
know where it is going.
So just to go back to the particle experiment, what does happen when you fire a high energy election at a proton?
That's the kind of thing which is easier to discuss.
Again the following is probably wrong, but when you fire an electron you create a current so we are getting towards
thing like the left hand motor rule.
http://en.wikipedia.org/wiki/Fleming%27s_left-hand_rule_for_motors
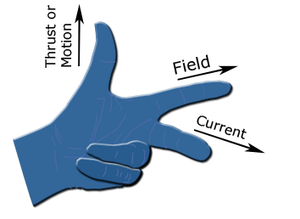
So I said this is all a bit wrong because maybe it is the corkscrew rule but what I am getting at which is probably don't wrong
anyway is that if you fire an electron at a proton, don't you get a force at right angles which would make it impossible to
hit the proton anyway?
Again as I said probably all wrong but can someone see what I am getting at and explain why I am wrong, preferably
start from where I am at rather than from somewhere else, if you see what I mean.
Anyway I will leave it at that for now.
0 -
That's just completely and utterly wrong. Electrons can't orbit at all because orbiting requires constant acceleration and if an electron did that it would radiate all of it's energy away, and I did some research
http://hyperphysics....base/uncer.html
And if you look at the 3rd box, the energy needed to force an electron in the nucleus is massive, I can see why it would take a huge particle accelerator.
There's a few theories for particles not falling into the nucleus. Some of them depict them as fields just simply don't exist in a way to interact in the nucleus, some picture them as waves who's probabilities don't exist enough in the nucleus nucleus, some theories view electrons as having odd extra-dimensional properties that make it move in dimensions we can't see.
Gravity and electro-magnetism are not the same thing, they have completely different equations and different force carrier particles.
Well as I said all theories are completely and utterly wrong when it comes to this sort stuff, so I can equally
say the theories you describe are completely and utterly wrong but that does not get he discussion anywhere does it?
I said we were in the realm of particle physics and I never said gravity was the same as electromagnetic force
just that they were both force.
I was just illustration a few points that even in the classical model the chances of hitting a proton are remote.
Anyway it goes on to say that that energy cannot be confined in the nucleus because a nucleus cannot
contain that energy but it does not say what happens.
Furthermore as you cannot say where the electron is due to the uncertainty principle how can you
say it does not orbit? How can you be so sure? You cannot say where the electron is so how
can you say it is not orbiting? That's a contradiction?
So I think you have to accept that according to the link you provided you cannot be certain the electron
is not orbiting, so you are wrong on that aren't you?
You are happy to call me 'completely and utterly wrong' so perhaps you will be big enough to
admit you are 'completely and utterly wrong' on that. - I doubt it some how, I really do....
Esbo, it is intuitive to say what you have just said. It is however common knowledge within Modern and Theoretical Physics that what you have stated is by today's standards incorrect. In fact the reason that QM exists is because we do not observe what you have just describe. In terms of particle interactions if this were true we would observe in experiments what is known as the Ultraviolet Catastrophe, which I will leave for you to research as there are ample discussions on the topic already. In brief observations with respect to blackbody radiation, as well as the photo-electric effect demonstrate that electrons occupy quantized energy levels around the nucleus, quite unlike what is observed with gravitational orbits.
** I was the one who gave you at the very least the first neg rep, I think it is savy that if you are to post in a thread like this you come prepared with at least a basic knowledge--as a minimum standard. I note this as you directed your comment to the mods after I had done so, and it wasn't them!
Well thanks for your courteous response

I think one or two of you may have misread my post because you seem to missed where I said "even if I am technically incorrect accord into
current theory" so some (one) of the attacks on me for being wrong are a bit over the top because I was the first to admit I was wrong.
So for Questionposter to say I am completely and utterly wrong he must be saying I am right, as I said I was wrong?
I will leave it for others to work that one out!!!
Thanks for the negative rep, I don't take much notice of reputations myself, I am rather more concerned with facts and models and science
than personal reputations, it is a very unscientific method to just correctness of someone by their reputation, that is why I do not use
it myself but each to their own, it takes all sorts as they say.
I do have a basic knowledge. I have A level physics at grade B, which would get me onto most university physics courses, that is more than most.
You say you do not observe what I describe but to be fair you are not sure what you are observing, so how can you say, "well it is not that"
when you can't say what it is?
See the problem is the scientist do all these experiment and work out all these various laws and then find they
do not actually fit together very well
0 -
I have been looking at the structure of the atom lately and wondered what makes the electron orbit? You would think if a proton is positively charged and a electron is negatively charged that the two would eventually stick together. I realize in theory that the orbiting electron like a planet never lets this happen. But what makes an electron orbit in the first place and when an electron goes from one atom to another how does it automatically orbit in a way that it does not collide with the proton or neutron? I am not interested in theories (there are way to many of them flying around), but in proof + experiments on what is going on.
Thanks.
Yea there is a force of attraction between and electron, but there is the same force of attraction between the earth and the moon, so would
you ask why the earth is not pulled into the moon?
It's basically the same question except the force is gravity not electro static but it is essentially the same 'problem'.
So the answer is the electron is falling towards the electron just as the moon is falling towards earth, without that falling the moon
would fly off into space or the electron would fall away from the proton.
I guess it the electron did hit the proton it would become a neutron, however once it is an electron in orbit it will never hit the proton
same as the moon will never hit the earth.
Even using that model, say another planet passed by earth and pulled the moon away, is it likely the moon would crash into
that planet? I think that is fairly unlikely.
A hydrogen atom is 100,000 time the size of the nucleus so the chances of it hitting are tiny.
Who is to say they do not hit, can we detect one neutron in 100,000 hydrogen atoms?
I don't really need another model to understand why it does not hit really, even if I am technically incorrect accord into
current theory.
So with that basic model you would expect the electron to orbit all the time and would be very surprise it it hit the nucleus.
You seem to be under the impression a collision is likely, which is understandable as there is a force of attraction, but the 'trick'
is unless it is aimed directly at the centre it will accelerate towards the proton, but it will be going so fast it will over come the
force of attraction and loop round into and orbit which will never collide with the proton.
Now if you want an experiment it woudl be hard to do wouldn't it?
Basically you would need a particle accelerator firing electrons at protons.
Then you are in the realm of sub atomic physics and strange particles etc..
http://www.emsb.qc.ca/laurenhill/science/quark.html
In 1935, the Japanese physicist, Hideki Yukawa, first proposed the existence of a particle responsible for a strong force. The discovery of the pi meson confirmed his hypothesis and won him the Nobel Prize in 1949. But the existence of quarks was only confirmed about 20 years later by Taylor, Friedmman and Kendall, who fired high energy electrons from a linear accelerator at protons and neutrons. Strangely, electrons were deflected at large angles. Sixty years earlier, Rutherford had obtained similar deflection angles upon firing helium nuclei at gold foil. The trio's results suggested that neither the proton nor the neutron was a solid sphere. Two up quarks and a down quark of charge +2/3 and -1/3, respectively, make up the proton, and two downs and an up make up a neutron.[note] to any over zealous mods (I know they exist sometimes) I am just throwing out some ideas out for
discussion not presenting a paper to the Royal Society, please don't pull me up on 'the rules' or whatever.
I am just putting out a model which like all models, is wrong.
 -2
-2 -
"every two of these problems" - does this mean any combination of two problems?
and is the number of contestants totally unconstrained, limited, or fixed?
update - the number of contestants must be limited. with 15 it is easy and clearly possible to show the opposite is true
Yes I remember finding it difficult to understand, it's every pair.
There is no limit on the number of contestants specified so there isn't an.
I recall the answer is achieved by showing a contradiction between some equation you derive
and the number of contestants does not come into it.
Helps to see here http://www.artofproblemsolving.com/Forum/viewtopic.php?f=267&t=44575&start=0
But it's tough to understand.
0 -
i'm not aguing with you; for picking 5 items from a group of 20, you are correct, 15504 is the number. however, i am considering the number of ways you can get a group of 10 items from 20. i think this is a better representation of the problem, as though person A has 15504 choices for selection, person B must choose from the 18476 choices to eleminate as many of person A's choices as possible, and each choice of 10 will eliminate roughly 252 choices. i could obviously be wrong with my "logic" here, it's a gut call. <BR>(to put it a bit more succently, i'm trying to factor in the overlap problem.)
Yes it is the overlap where the fun really begins.
I remember trying a similar problem, I will see if I can find it, the solution to the problem was also given I think.
Ah!!!! I have found it!
In a mathematical competition, in which
 problems were posed to the participants, every two of these problems were solved by more than
problems were posed to the participants, every two of these problems were solved by more than  of the contestants. Moreover, no contestant solved all the
of the contestants. Moreover, no contestant solved all the  problems. Show that there are at least
problems. Show that there are at least  contestants who solved exactly
contestants who solved exactly  problems each.0
problems each.0 -
Are you completely sure you know what factorials are? I guess I could be looking at it the wrong way though.
Yes I know what they are ie 4! = 4x3x2
This is about perms though and it's more complicated than just perms I believe.
for example consider these two
11111111110000000000
01111111111000000000
Looks nice consider the first two line - but you miss the this line of 5
11111111110000000000
01111111111000000000
10111000001000000000
using an ugly "brute force" attack, i get the number of nessicary groups of 10 to be at most 2540.
I expect the answer is ugly, what was your attack?
A computer program?
Even that would require a lot of though on the strategy???
Or maybe I am missing something?
I suppose you could do all the number of perms of 10 from 20 and get an answer X
then find all the perms of 5 from 10 and multiply by X
But you may get loads of duplicates?
0 -
Mmmmmm nasty problem, I remember seeing something similar to this but with smaller sets, can't remember exactly immediately.
Not sure if there is an easy way to solve it.
As imafall says "you need at least 62 groups of 10 if your coverage is perfect - whether that is possible I do not know yet."
I would guess it is not possible.
Starting from a simpler example, say a person picks a set of 1 from 3, now how many sets of 2 do you need to cover all the sets of 1?
001
010
100
110
011
answer 2!!
Now try 2 from for covered by 3 from 4.
0011
0101
1001
1010
1100
0111
1110 so 2 sets nearly does it but it misses:-
1001 so you need a 3rd set 1101 or 1011.
For the original problem suppose you go
11111111110000000000
00000000001111111111
Well that covers two halves but big problem for those straddling the two halves!!
It is at this point the head spinning starts.
0 -
The problem reoccurred which I put down to change in weather conditions perhaps so I decide to try your suggestions.
Unplugging the sound made no difference.
Then I tried the video but then realised I could not see the screen to play the video anyway whilst fiddling around
with the connection the problem seemed to go away, but further fiddling 'killed' the video card, so I had to reboot and hope
it still worked, which thankfully it did. The card is rather loose in it's slot, it's has no securing screw so it's a bit dodgy
messing around with it so I will leave it as it is.
Anyway the problem seems to have gone unless the climate conditions improved suddenly.
Still rather puzzling how one video seem to cause interference, maybe it just happens to set up
or hit come resonant frequency. I suspect it may be something to do with the black box round the
video.
0 -
I went to Radioshack and the voltage rating is not on there. I even ask the front desk worker and even he doesn't know
the front desk work probable knows little more than to take the money and handover change
and a receipt, don't expect an engineering mastermind.
0 -
basically with a transistor, the carrier signal fed across a transistor and the message signal applied to the base thus controlling the amplitude
of the carrier making it proportional to the message.
A similar method can be used to control the frequency of the carrier signal, the advantage is the carrier signal is always full strength
so you don't get noise problems with quite bits in music for example/.
click the green + button
 1
1 -
It's not happening today, mind you I have moved the TV a bit.
Different weather seem to have improved reception overall.
0 -
Bit of a weird one, I have terrestrial digital TV though and aerial and booster.
I noticed some interference on the TV ('blocking') as i was playing a youtube video.
However when I paused the video the interference stopped and I found I could
cause or stop interference by pausing or playing the video!! Weird I thought.
The signal is not great on this TV channel but it usually OK.
I also tried playing a video in VLC player but that caused no interference?
Even weirder I tried other youtube videos and they did not seem to cause
interference, certainly not anywhere near to he same extent.
The video in question was.
I even tried another U2 video which I think was from the same concert and that cause no or little
interference!!!
I tried playing it with the sound and monitor turned off and it still cause interference!!
How weird is that!!
I though it might be because the video is boxed but it occurs full screen and in different sizes.
It does seem to help if I minimise the window seems to reduce it by over half.
Strange eh?
Bit creepy especially as Bono dedicates this song to his recently deceased dad.
This is not a wind up!! I wish is was!! I will try it again tomorrow.
Also it does not seem to do it playing the above embedded video
Which was ht tp://www.youtube.com/watch?v=uDkBzkA9L4s
I put a space in to stop it being embedded automtically
0 -
Does religion (or just believing in anything supernatural) have any advantages for surviving? When you are looking at it from a point of natural selection?
Why is the man religious?
I would say that without religion there is no society.
If you look back al the earliest evidence of civilisation is religious based stuff, name me a early civilisation that was not religious?
Without religion people turn into savages and that destroys society, I think we see a lot of that.
But having said that religion is not about this world it is about the next.
Having said that rats and cockroaches are not religious an they do pretty well.
For humans the morality inherent in religion makes for a better society, natural selection is often misunderstood
people use it to justify selfish and barbaric and immoral behaviour. People use it to justify their bad behaviour,
it is a dangerous idea in the hands of the stupid, it seems simple but it is actually very complex.
0 -
Maybe there is no available chemical that would facilitate absorption of all wave lengths of light.
There are chemicals which will absorb all colours and a composite of those chemicals would absorb all colours.
0 -
Do you enjoy the fact that when you join a forum you become a second class citizen denied free speech
and freedom to choose what you read?
So can we take an a vote on this ie indicate is you prefer censorship or freedom of expression?
So vote either "freedom" or "censoeship" at the end of your post (or otherwise).
Also give your reasons if you wish too.
My vote - freedom!!!
 -1
-1 -
Natural Selection. The green has survived to overall dominance because it's the most robust in terms of fitting in with the greatest variety of environments and therefore promoting its reproduction. Black and red are colours that only 'fit' in narrower and more specific environmental niches, therefore, they are much less populous. Simple.
I think you are somewhat simpler than your answer!!
Again the question is not being answered, you simply avoided it.
You have to explain why they are green in terms of specific factors, in detail showing you understand the factors involved rather than
omitting those factors. So not quite so simle.
I think that question has been answered, red and blue light power chlorophyll so the plants reflect green light. In environments where the light spectrum is significantly different other pigments have evolved but they work with chlorophyll. Chlorophyll's resemblance to other organometallic compounds commonly used by life suggests that chlorophyll came about because it was easier to modify existing compounds chemosynthesis was already based on rather than start over from scratch. I don't see why you can't see that...
I am afraid that answer is not good enough because plants grow in an environment where there is blue red and green light, not just blue and red.
The rest of your answer is flawed, full of asumptions you have not proven.
-2 -
They're green because the trait of being green has survived, and other traits like black or red or blue haven't so much.
Now all you have to do is explain why that happened, then you will have answered my question.
Basically all you have done is turned my question into a statement rather than answering it.
Now I will turn your statement back into the original question

"Why has the trait of being green survived, and other traits like black or red or blue haven't so much?"
Now try and answer that one!!!!!!!!
 0
0 -
So you're saying that you're ok with evolution but not speciation?
No selection is not evolution it's just selection, nothing is evolving we are just
witnessing extinction, which is nothing new.
If you want to say that over times many animals have become extinct I
am perfectly happy with that, because there is some evidence for it.
The problem for evolutionists is that we fail to see all the new animals it should be producing
that is why I feel the case for evolution is not there, the evidence is not there.
That is the problem for you evolutionists, you have blind faith in it but sadly not the new animals
it should produce if it worked.
Natural selection has a specific definition in a scientific context - you can't change the definition of things to suit your specific argument - this is called a strawman argument and is a logical fallacy.
http://en.wikipedia....tural_selection
Unfortunately it is science which is changing the definition of things, ie it is turning 'theory' into 'law' inappropiately.
The fact science has to redefine so many words to make it work should set the alarm bells ringing for you.
You have elevated scientists into a god-like status and just blindly follow the dogma, failing to question why
it needs to redefine words, this also makes it difficult to discuss when science has changed the meaning of word
away from the true meaning in order bamboozle it's critics.
Changing the meaning of words is the hallmark of a crook and a conman.
-2 -
Natural selection is a basic process of evolution.
No natural selection is just natural selection ie any kind of selection from a group of thing,
ie if I select fish and chips from the menu that is my natural selection, it's a long way
from evolution, the chips have not turned into a turnip and the fish has not turned into
a turkey.
0 -
I don't understand how you can simultaneously believe in natural selection and not evolution.
Because it is selection within the same species, you are never gonna naturally select an elephant from a butterfly,
that just aint gonna happen.
Ginger haired people are never gonna evolve into a different species.
That's just ludicrous

This is what you lot fail to understand, variation within a species is limited.
That is why evolution had never been observed or reproduced in the lab.
Until it has it remains a theory because a theory must be proven before it comes a law.
On and by the way the link to 'scientific theory' in wikipedia is unverified, I think some of
you are being mislead by someone hacking wikipedia and putting in false unverified definitions.
http://en.wikipedia.org/wiki/Scientific_theory
This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. (May 2009)0 -
I don't understand how you can simultaneously believe in natural selection and not evolution.
Because it is not evolution, it is the same species it is just variation within the species, following your
logic black man are a different species from white men, do you you seriously expect people to believe that??
It's also slightly racist.
.
-1 -
That's natural selection.

but not evolution!!

I will go through the rest of the stuff later.
 -1
-1



What makes an electron orbit?
in Modern and Theoretical Physics
Posted
Well what happens is they do an experiment and then come up with a theory to explain what is happening, however that does not mean
the theory is correct just that it seems to explain what is happening. Often however the theory turns out to be wrong when further
experiments are done, as happened with a lot of classical physics being out dated by all this relativity stuff.
I mean there is all sorts of stuff which has been over turned by new developments.
I recall one physicist saying "well this theory has been around for 100 years now so we can be fairly confident it is correct!!"
(I forget what he was talking about). However I think he needs to recognise that in the past there were theories which had been
around for thousands of years before they turned out to be wrong!!