

kristina221
-
Posts
18 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by kristina221
-
-
I wondered if I could do an Aldol condensation of ninhydrin and acetone. I accidently put too much KOH in acetone and when I added ninhydrin in, the blue colour appeared. I tried it again with just ninhydrin in base and got the same result. When I grinded some ninhydrin(s) with KOH(s) in a mortar the mixture turned dark blue and some liquid appeared. I tried putting some oxalic acid in the blue solution and there was no change in colour, but when I added H2O2(30%) in another sample of blue solution CO2 formed.
So I could say that the purpose of this experiment is satisfying my curiosity
I did try googling it, but all I got were posts related to the reaction of ninhydrin with aminoacids
 0
0 -
I've put some undissolved ninhydrin in a test tube and added about 0,5 mL of conc. KOH(about 5 M). The colour changed to dark blue. I tried the same thing with ninhydrin(aq) and nothing happened.
When I added H2O2 to the blue solution, CO2 started bubbling from the solution( I know it is CO2 because I tested it with Ca(OH)2 (aq) and it turned cloudy white, and after adding HCl turned clear again).
Is it possible that H2O2 oxidized that blue compound to phthalic acid?
What would be the structure of that compound?
And can anyone propose a mechanism?
0 -
I believe that the first step would be deprotection of the keto-group and that the products would be acetone and a diol. The next step would be formation of a cyclic ester (lactone). There are two possible products and I would go with the one that has less strained ring.
0 -
Alice in chains, The 69 Eyes, Metallica, The Sisters of Mercy, Opeth, Enya, Bauhaus, Pink Floyd, RHCP, few Nightwish's songs, Tristania, Vivaldi, Disturbed, Apocalyptica, Maksim Mrvica, Moonspell, Nirvana, Pearl Jam, Sirenia, Simon & Garfunkel, Siouxie & The Banshees...
0 -
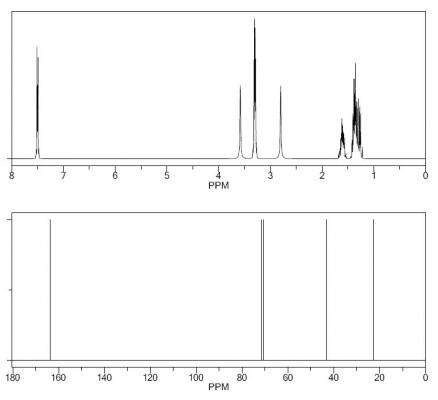
i assume that the 160 and 7.5 shifts are for the imine c atom
0 -
why not just heat up a teapot full of water , throw it on the ice and move your car before it freezes again?
0 -
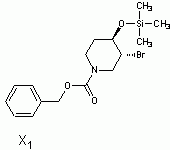
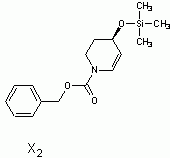
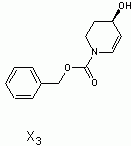
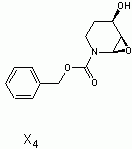
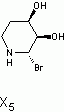
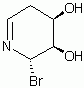
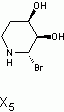
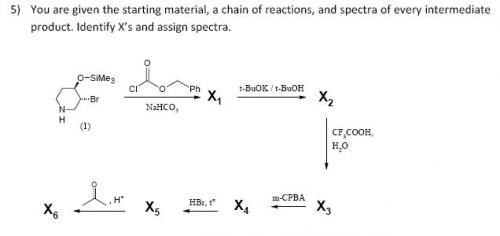
in the end i decided to go with (3S,4R)-3,4,5,6-tetrahydropyridine-3,4-diol as x5
0 -
my favourites:
You build little worlds, little stories, little shells around your mind and that keeps infinity at bay and allows you to wake up in the morning without screaming!
– A Hat Full of Sky
SOME PEOPLE WILL DO ANYTHING FOR THE SHEER FASCINATION OF DOING IT, said Death. OR FOR FAME. OR BECAUSE THEY SHOULDN'T.
– Hogfather
HUMANS NEED FANTASY TO BE HUMAN. TO BE THE PLACE WHERE THE FALLING ANGEL MEETS THE RISING APE.
– Hogfather
The thing about words is that meanings can twist just like a snake, and if you want to find snakes look for them behind words that have changed their meaning.
– Lords and Ladies
all by Terry Pratchett
0 -
When we ignated a bit of picric acid(about 1 g) it burned through the asbestos heating net...
0 -
I would be extremly suprised if that proton was that high. Protons above 7ppm are either aromatics or they are aldehyde (unless there are some interesting ring systems). Can you put what you propose for X1-6 so we can see if your along the right lines. I've come up with a structure but I want to see what you;ve got so I can give you the helping nudges.
I'm aware of that fact. That's why I'm asking
 0
0 -
All from the above + Boccaccio: Decameron
And that's only the tip of an iceberg...
0 -
Neil Gaiman: American Gods, Ray Bradbury: Fahrenheit 451, M. Zusak: The book thief, W. Golding: The lord of the flies. I also liked when we read Boccaccio: Dekameron and Pirandello: Six Characters in Search of an Author in school. I'm half-way through Organic Chemistry by Clayden, Greeves, Warren and Wothers.
0 -
Picric acid, aresenic and cromyl chloride(CrO2Cl2). The last one gave me a headache that lasted for 2 days...
0 -
Here is the spectra of compound X5 and what I think might be X5. Peaks at 163 ppm (13C) and 7.5 ppm(1H) are the fact that is confusing me. No, there is no molecular formula or MS. I understand the last step, but I'm confused by X6 nmr too, because those peaks appear again. The only explanation I came up with is that the H on the carbon atom between the OH and Br is so strongly deshielded.
Or is it 2-bromo-2,3,4,5-tetrahydropyridine-3,4-diol
0 -
in that case i think the soot could act as a black body to protect the plants from radiation(probably UV)
0 -
"Fog or smoke Clouds and fog are well-known for their ability to reduce radiative heat loss from the surface. Smoke from smudge pots or burning tires or refuse and mist from fine water nozzles have been used in attempts to reduce this heat loss. Since it is difficult to maintain the smoke over the sensitive crop area and to produce droplets the optimum size to intercept the long-wave radiation, this method is not very effective. In addition, our environmental laws now prohibit the use of this method, where smoke is involved."
from http://www.omafra.go...5-116.htm#freez
and...
"A smudge pot (also known as a choofa or orchard heater) is an oil-burning device used to prevent frost on fruit trees.
Condensation of water vapor on particulate soot prevents condensation on plants and raises air temperature very slightly."
From the Wikpedia Smudge Pot article.
thank you, that helped

other ideas are also welcome...
0 -
This is not a homework assignment, but I didn't know where to post it.
I found this question in a book about physics, but there was no explanation. Q: Why do fruit fruit growers set car tires on fire in their orchards in the clear winter mornings?
I imagine te answer would be to stop frost from forming, but I don't understand how smoke or soot could do that. The chapter is about wave-like properties of electromagnetic radiation and particles. Could soot act as a black body at 32°F (273 K)?
0

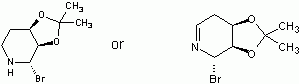
Conferences and Science Events - Links
in Science Education
Posted
43rd International Chemistry Olympiad. More information at http://icho43.metu.edu.tr/