-
Evaporation and condensation as a source of energy
I was wrong. Sorry. Thank you for your attention.
-
Evaporation and condensation as a source of energy
sethoflagos On this sunny Lagos afternoon... BestChance Everything is simple. The diagram C-A shows what happens to the piston in fig. 3. Now let's let the piston go down. Under the action of the piston, the cooled mixture of gaseous and liquid carbon dioxide will pass into tank B. When the piston reaches the minimum stroke, close valve B and open valve A, see fig. 3 The diagram A-B - what happens to the piston in fig. 1. Liquid carbon dioxide at ambient temperature, in "liquid - vapor" equilibrium, from tank A entered the tank with a piston, see fig. 1. After receiving the required amount of carbon dioxide, valve A was closed. Then the piston, under the action of the pressure force of saturated carbon dioxide vapor on it, made its upward stroke, where it was stopped when it reached its maximum stroke. In this case, the adiabatic expansion of gaseous carbon dioxide under the piston and the boiling of liquid carbon dioxide at the bottom of the tank occurred, followed by a decrease in their temperature and pressure. Then the valve B open, see fig. 2. The diagram B-C - what happens in fig. 2. Now let's let the piston go down. Under the action of the piston, the cooled mixture of gaseous and liquid carbon dioxide will pass into tank B. Then again C-A. Everything matches. As for the high cost, do not tell my slippers. TheVat Isn't Bestchance a sock puppet of Erik2014, and this is a clone of this earlier thread? BestChance Let's leave Eric2014 and 2014 alone. Now is not up to it.
-
Evaporation and condensation as a source of energy
exchemist No. You have 2 tanks at different temperatures. Only one of these can be at the" ambient" temperature of the environment. The other must be at a different temperature, either higher or lower, in order for the engine to work. How is that different temperature created? BestChance Carbon dioxide from tank A at ambient temperature enters the tank with the piston. Valve A is closed. The piston goes up, the volume occupied by carbon dioxide increases. The temperature of carbon dioxide decreases due to adiabatic expansion. Valve B opens. The piston goes down. In a cooled state, carbon dioxide enters reservoir B. In short. Different temperatures are created due to the adiabatic expansion of carbon dioxide. studiot So what is the point of the thermal insulation ? Please complete your engine description without missing stuff out. BestChance The thermal insulation of the piston tank is necessary for the adiabatic expansion and therefore for the cooling of the carbon dioxide under the piston. Thermal insulation of tank B is needed so that carbon dioxide can freely enter tank B from the tank with the piston. Thermal insulation of tank A is optional, just so convenient for logical analysis. The indicators are constant.
-
Evaporation and condensation as a source of energy
studiot As I understand your idea, you want to extract thermal energy (heat) from the environment into your engine where that energy is converted and output as work. Quite right. studiot Yet you also say that the system is insulated. Quite right. studiot So, first question, how does the heat from the environment enter your engine ? It's simple. If necessary, the cooled tank can be brought into contact with the environment. Then the thermal insulation can be restored.
-
Evaporation and condensation as a source of energy
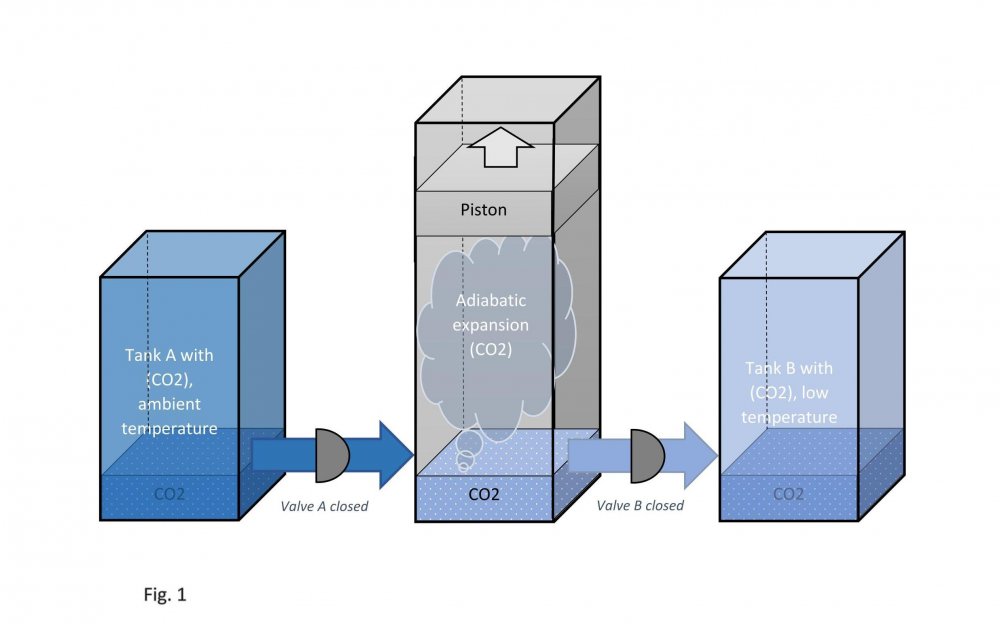
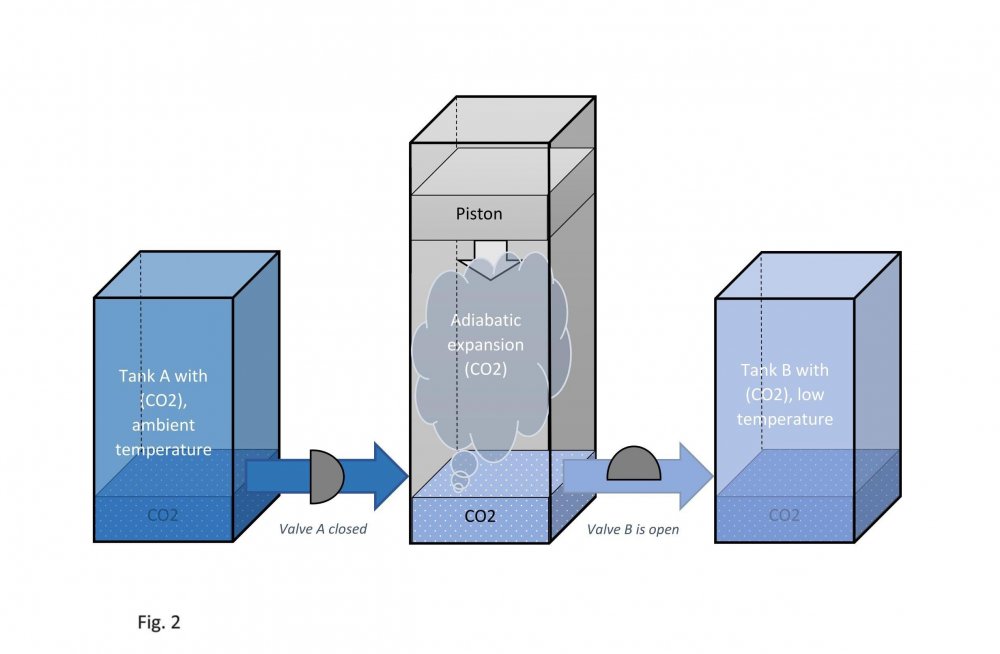
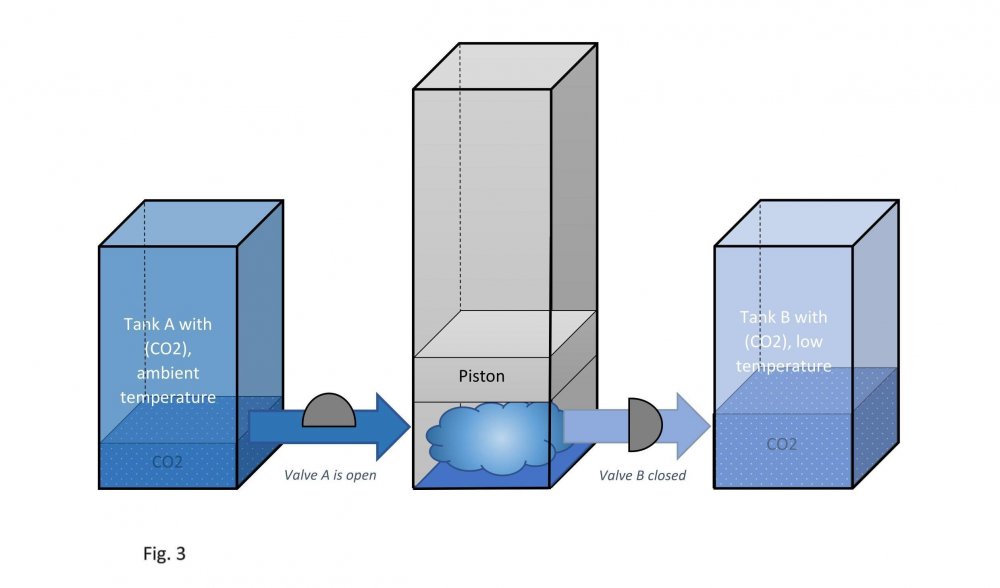
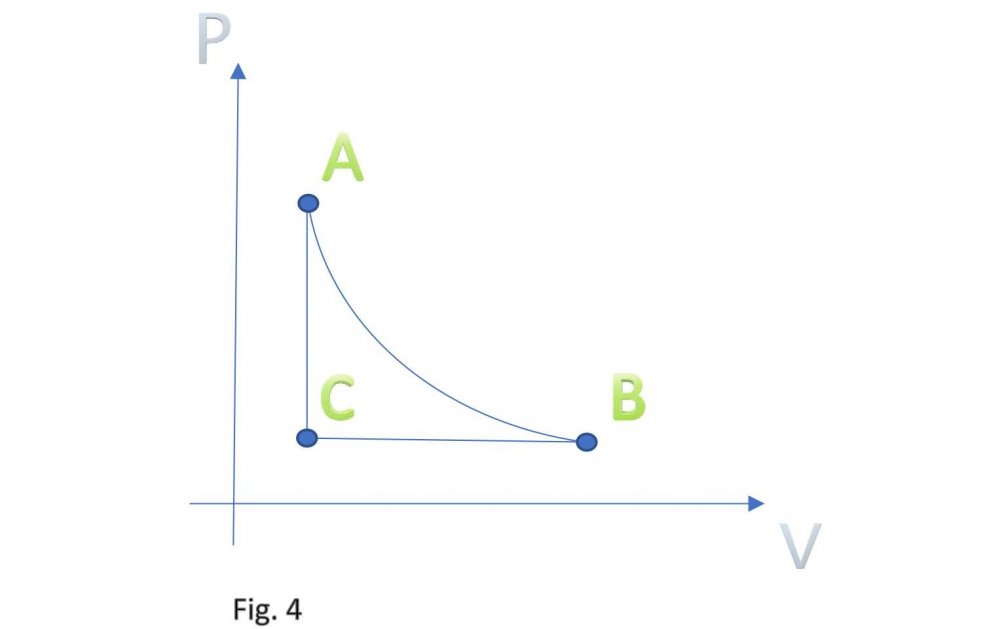
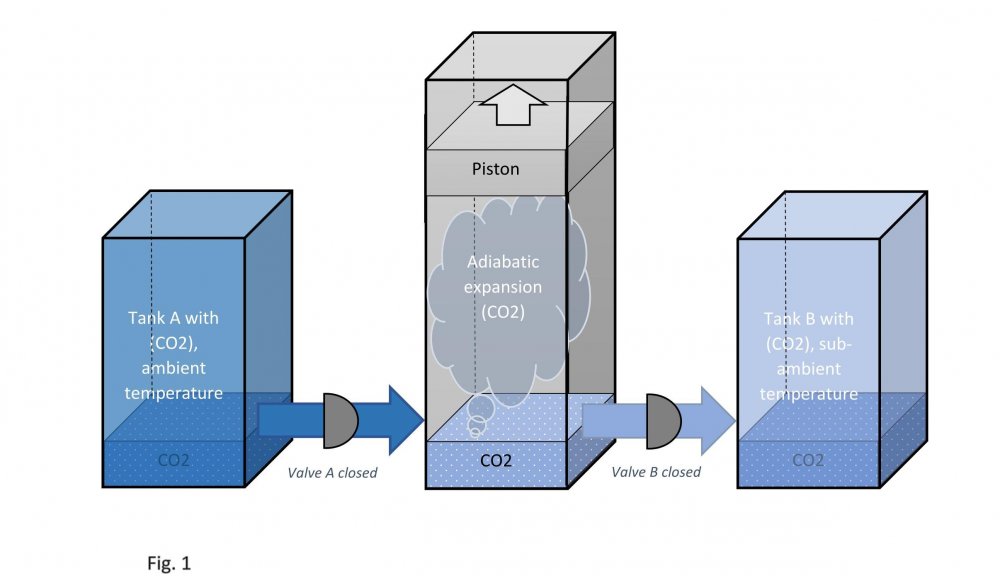
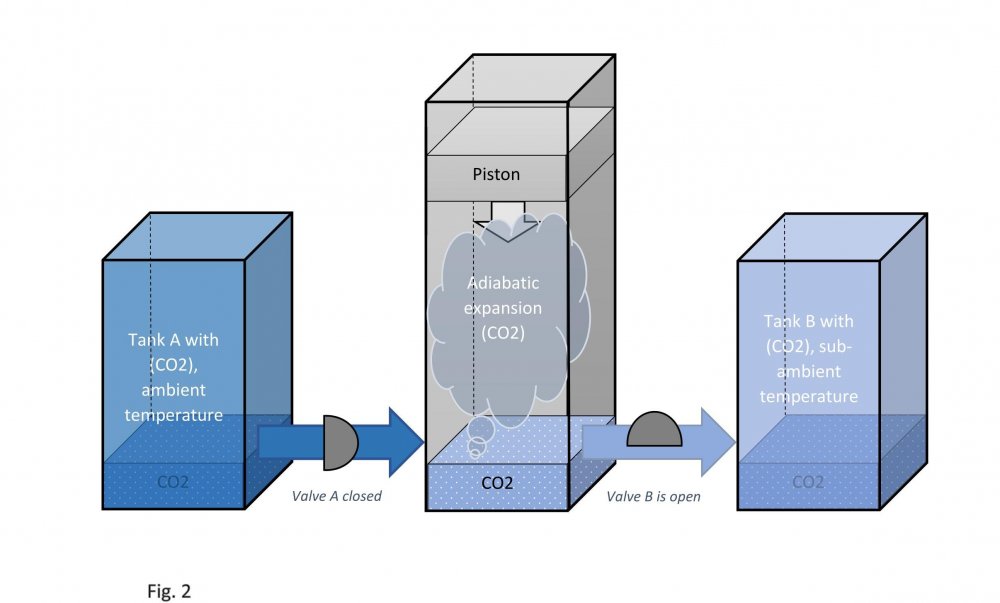
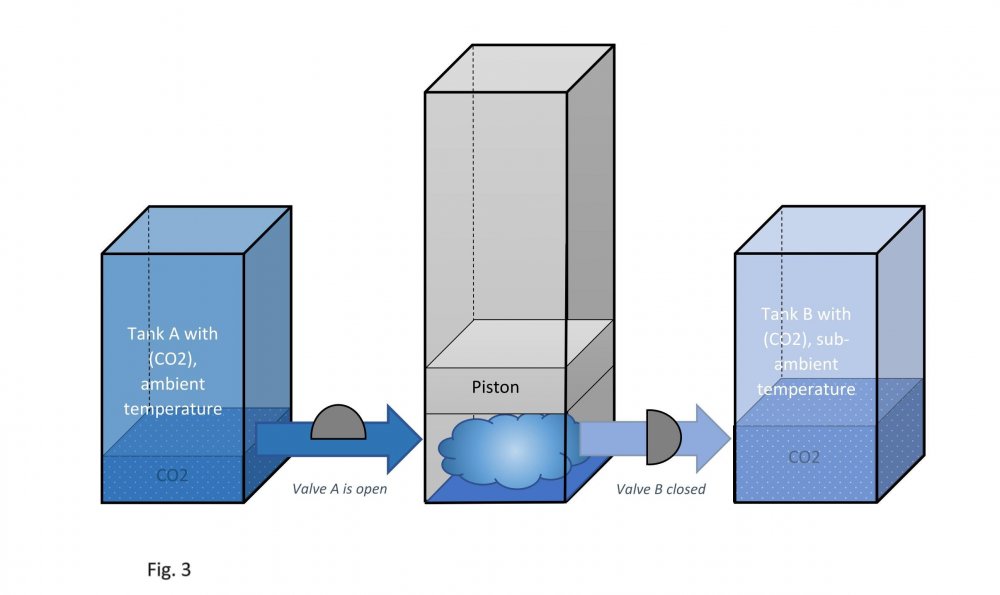
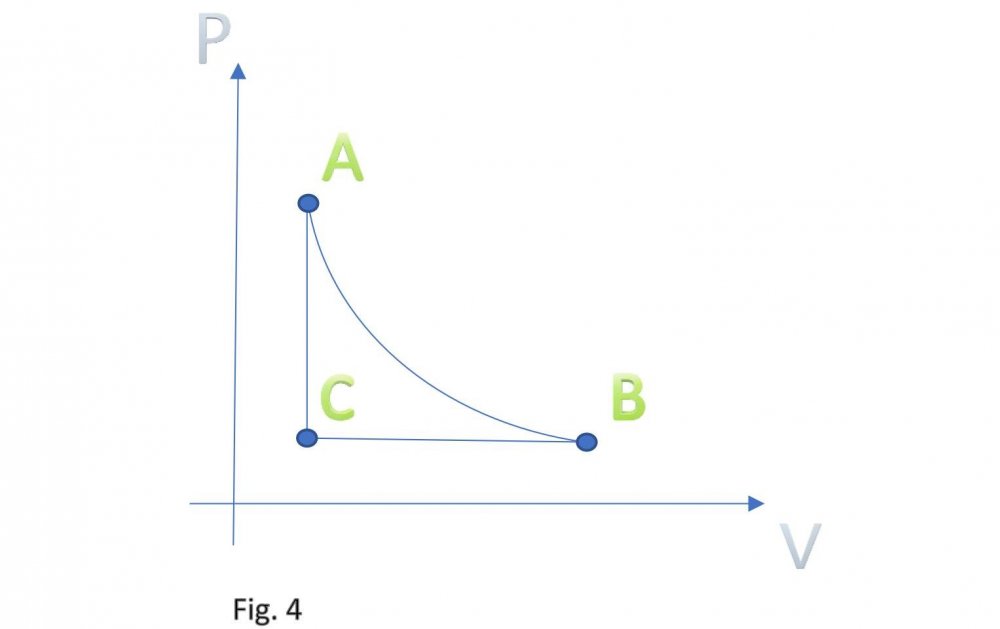
It is assumed that the temperature of carbon dioxide under the piston is equal to the temperature of carbon dioxide in tank B. This means that the pressure under the piston during its downward stroke is equal to the pressure in tank B (low temperature). Actually, I don't know why google translator translated low temperature as "sub-ambient". Actually, I don't speak English at all. Edited fig. 1, 2 and 3 Dedicated to those who do not accept war Evaporation and condensation as a source of energy Your attention is presented to the concept of extracting the thermal energy of the environment based on the processes of evaporation and condensation of a substance that is in equilibrium "liquid - vapor". In our example, this substance is carbon dioxide, we will denote it in the figures as (CO2). Three heat-insulated sealed tanks are shown below: tank A with liquid carbon dioxide at ambient temperature, tank with piston and tank B with liquid carbon dioxide at sub-ambient temperature. The connections of tanks A and B to the piston tank are controlled by valves A and B, as shown in fig. 1, 2 and 3. The flow of liquid and gaseous carbon dioxide from tank A to piston tank and from piston tank to tank B are shown by horizontal arrows in fig. 1, 2 and 3. Vertical arrows in fig. 1 and 2 show the piston strokes. Fig. 1 Liquid carbon dioxide at ambient temperature, in "liquid - vapor" equilibrium, from tank A entered the tank with a piston, see fig. 1. After receiving the required amount of carbon dioxide, valve A was closed. Then the piston, under the action of the pressure force of saturated carbon dioxide vapor on it, made its upward stroke, where it was stopped when it reached its maximum stroke. In this case, the adiabatic expansion of gaseous carbon dioxide under the piston and the boiling of liquid carbon dioxide at the bottom of the tank occurred, followed by a decrease in their temperature and pressure. Then the valve B open, see fig. 2. Fig. 2 Now let's let the piston go down. Under the action of the piston, the cooled mixture of gaseous and liquid carbon dioxide will pass into tank B. When the piston reaches the minimum stroke, close valve B and open valve A, see fig. 3. Fig. 3 The required amount of liquid carbon dioxide from tank A will again pass into the tank with the piston. Valve A is closed again. Then the piston, under the action of the pressure force of saturated carbon dioxide vapor on it, will again make its upward stroke, where it will be stopped when it reaches its maximum stroke. The position of the reservoir system and the piston will be as it was at the beginning, and is shown in fig. 1. The piston, in its full stroke from bottom to top and back, does work that can be diverted to the consumer to generate, for example, electricity. This useful work on the P-V diagram, see fig. 4 below, is shown as the area of the figure bounded by lines converging at points A, B and C. Fig. 4 The diagram C-A shows what happens to the piston in fig. 3. The diagram A-B - what happens to the piston in fig. 1. The diagram B-C - what happens in fig. 2. For a number of full stroke cycles, from bottom to top and back, the piston fills reservoir B with cooled liquid carbon dioxide. A full tank B of chilled carbon dioxide can be used as a refrigerator or other useful uses. When the temperature of tank B equalizes with the ambient temperature, tanks B with liquid carbon dioxide and the emptied A, can be swapped to restart the piston. In this way, humanity can make up for the lack of energy for its needs and, at the same time, ensure the ecological balance of its planet. This is a real chance. Emil Kutin January 22, 2022, Russia, St. Petersburg
-
Evaporation and condensation as a source of energy
Dedicated to those who do not accept war Evaporation and condensation as a source of energy Your attention is presented to the concept of extracting the thermal energy of the environment based on the processes of evaporation and condensation of a substance that is in equilibrium "liquid - vapor". In our example, this substance is carbon dioxide, we will denote it in the figures as (CO2). Three heat-insulated sealed tanks are shown below: tank A with liquid carbon dioxide at ambient temperature, tank with piston and tank B with liquid carbon dioxide at sub-ambient temperature. The connections of tanks A and B to the piston tank are controlled by valves A and B, as shown in fig. 1, 2 and 3. The flow of liquid and gaseous carbon dioxide from tank A to piston tank and from piston tank to tank B are shown by horizontal arrows in fig. 1, 2 and 3. Vertical arrows in fig. 1 and 2 show the piston strokes. Fig. 1 Liquid carbon dioxide at ambient temperature, in "liquid - vapor" equilibrium, from tank A entered the tank with a piston, see fig. 1. After receiving the required amount of carbon dioxide, valve A was closed. Then the piston, under the action of the pressure force of saturated carbon dioxide vapor on it, made its upward stroke, where it was stopped when it reached its maximum stroke. In this case, the adiabatic expansion of gaseous carbon dioxide under the piston and the boiling of liquid carbon dioxide at the bottom of the tank occurred, followed by a decrease in their temperature and pressure. Then the valve B open, see fig. 2. Fig. 2 Now let's let the piston go down. Under the action of the piston, the cooled mixture of gaseous and liquid carbon dioxide will pass into tank B. When the piston reaches the minimum stroke, close valve B and open valve A, see fig. 3. Fig. 3 The required amount of liquid carbon dioxide from tank A will again pass into the tank with the piston. Valve A is closed again. Then the piston, under the action of the pressure force of saturated carbon dioxide vapor on it, will again make its upward stroke, where it will be stopped when it reaches its maximum stroke. The position of the reservoir system and the piston will be as it was at the beginning, and is shown in fig. 1. The piston, in its full stroke from bottom to top and back, does work that can be diverted to the consumer to generate, for example, electricity. This useful work on the P-V diagram, see fig. 4 below, is shown as the area of the figure bounded by lines converging at points A, B and C. Fig. 4 The diagram C-A shows what happens to the piston in fig. 3. The diagram A-B - what happens to the piston in fig. 1. The diagram B-C - what happens in fig. 2. For a number of full stroke cycles, from bottom to top and back, the piston fills reservoir B with cooled liquid carbon dioxide. A full tank B of chilled carbon dioxide can be used as a refrigerator or other useful uses. When the temperature of tank B equalizes with the ambient temperature, tanks B with liquid carbon dioxide and the emptied A, can be swapped to restart the piston. In this way, humanity can make up for the lack of energy for its needs and, at the same time, ensure the ecological balance of its planet. This is a real chance. Emil Kutin January 22, 2022, Russia, St. Petersburg

BestChance
Members
-
Joined
-
Last visited