-
Huge Ideal Gas Law Problem!
Of course. You're absolutely right. Thanks so much!
-
Huge Ideal Gas Law Problem!
Hello everyone! I have been tasked with creating a airbag from a plastic sandwich bag, acetic acid, and sodium bicarbonate. The volume of my sandwich bag is 0.87 L, the pressure in my area is 100.6 kPa, and the temperature in the room is 295.15 K. First, I am tasked to find the mass of NaHCO3 needed. My calculations are as follows: n = PV / RT n = (100.6 kPa)(0.87 L) / (8.314 kPa x L / mol x K)(295.15 K) n = 0.03566682292 mol NaHCO3 M = 75.028 g/mol m = n x M m = 2.676 = 2.7 g Now, I need to calculate the volume of acetic acid, but I run into a roadblock: nHC2H3O2 = (0.03566682292 mol NaHCO3)(1 mol HC2H3O2 / 1 mol NaHCO3) nHC2H3O2 = 0.03566682292 mol V = nRT / P V = (0.03566682292 mol)(8.314 kPa x L / mol x K)(295.15 K) / 100.6 kPa V = 0.8666 = 0.87 L Huh? I doubt I need to fill the bag completely full with acetic acid to produce a reaction. What have I done wrong here? Thank you so much for the help!
-
Dilution Confusion
Hello again, everyone! Today, I present you all with a question about dilution and it's formula of C1V1=C2V2. I believe it's also common to see "M" in place of "C". My question is, "How many mL is needed to dilute a 100 mL solution of NaOH with a concentration of 10M to a concentration of 2M." I believe the answer is 20 mL: C1V1=C2V2 C1 = 10M (initial concentration) V1 = ? (this is the amount of solution that must be transferred, right?) C2 = 2M (desired concentration) V2 = 100 mL (not entirely sure why this value is 100 mL, but I think it is because that's how much I want to end up with?) (10M)(V1) = (2M)(0.1 L) V1 = [(2M)(0.1 L)] / 10M V1 = 0.02 L = 20 mL Now, I believe this means that 20 mL of NaOH should be diluted in 80 mL of water? This will give the NaOH a concentration of 2M and a final volume of 100 mL. Do I understand this correctly? Thanks a lot for the help
-
Please Check My Work :)
Thank you so much! I'll definitely make the change over to scientific notation. I am just so hell-bent on visualizing everything that it'll take a bit of practice. Have a great day!
-
Please Check My Work :)
C + O2 → CO2 mC = 2680 kg = 2680000 g mO2 =? mCO2 = ? MC = 12.01 g/mol MO2 = 2MO MO2 = (2)(16.00 g/mol) MO2 = 32.00 g/mol MCO2 = MC + 2MO MCO2 = (12.01 g/mol) + (2)(16.00 g/mol) MCO2 = 44.01 g/mol nC = mC / MC nC = (2680000 g) / (12.01 g/mol) nC = 223147.3772 mol nO2 = (223147.3772 mol C) (1 mol O2 / 1 mol C) nO2 = 223147.3772 mol nCO2 = (223147.3772 mol O2) (1 mol CO2 / 1 mol O2) mCO2 = nCO2 X MCO2 mCO2 = (223147.3772 mol)(44.01 g/mol) mCO2 = 9820716 g mCO2 = (9820716 g) / (454 g/lb) mCO2 = 21632 lbs Therefore, the mass of CO2 produced from coal is 21632 lbs every year per person. m = mass M = molar mass n = number of moles I am just a little thrown off by the fact that there isn't a limiting or excess reactant in this question. Every example I've done in class and in homework has included one. Since there isn't a limiting reactant, can I use the n-value of either carbon or oxygen to determine the n-value for carbon dioxide (since they're the same values)? I have 2 more questions like this that need to be checked if you guys are bored, let me know if that interests you!
-
Conversion Factor Question
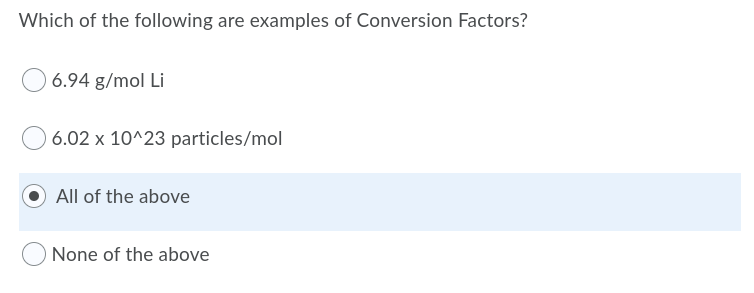
This is the exact question: I'm fairly certain 6.02 x 10^23 mol^-1 (Avogadro's constant) is a conversion factor.
-
Conversion Factor Question
I understand what it means. I just don't understand whether or not 6.941 g/mol is a conversion factor. The question is worded abhorrently. For example, 6.02 x 10^23 mol^-1 would be a conversion factor, but is 6.941 g/mol one? You use the molar mass of Li (g/mol) to determine either the mass (g) or amount of moles (m), right? So is that an example of a conversion factor? A value, paired with 2 units (in this case g/mol) that can determine the value of another unit?
-
Conversion Factor Question
Hey everyone! Super quick question. "Is 6.94 g/mol Li a conversion factor?" I'm just unsure of what the answer could be. I assume it is because you use the molar mass to determine the mass of a substance? Thanks for the help!
-
Understanding The Reactivity Series
Hello, everyone! I have a quick homework question that I assume has some relation to the reactivity series? It goes like this: "Would it be a good idea to store a solution of zinc nitrate in a container made of iron? Why or why not?" I suspect it would be a fine idea to store the solution of zinc nitrate in a container made of iron because iron is less reactive than zinc and cannot displace it? I'm not entirely sure, as we skimmed over the reactivity series in class, so I would really appreciate a more in-depth and thorough answer. Thank you guys so much!

Kagi98JP
Members
-
Joined
-
Last visited