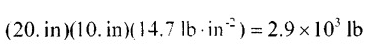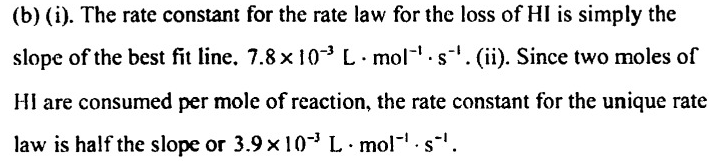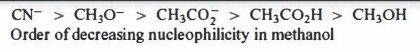Everything posted by Scienc
-
Entropy Calculation
For an ideal gas that expands against P = 0, is Delta T = 0?
-
Entropy Calculation
Hi everyone, I am studying thermodynamics, and I'm confused about item b. I understand why the professor used W = 0 since Pexternal = 0; however, I'm unsure why Delta T also equals 0. Should I consider that process B is also isothermal, or is the phrase "an isothermal reversible expansion" only applicable to item c? From what I recall, an expansion for Pexternal = 0 does not necessarily result in a Delta T = 0, or it does.
-
Gas Expansion Resolution
I believe the individual who solved this problem included a negative sign in front of the first equation (ΔU = nCpΔT) because they reversed the order of the temperatures in their calculations. The equation itself does not contain a negative sign.
-
Gas Expansion Resolution
Isn't it? Since the final temperature is 300 K and the initial temperature is 450 K, we can calculate the change in temperature (ΔT) as follows: ΔT = (Tf - To) = (300 - 450) = -150 K. By the way, I think the person who solved this problem included a negative sign in front of the first equation (ΔU = nCpΔT) because they used the temperatures in the reverse order in their calculations.
-
Gas Expansion Resolution
Is this resolution wrong? Since the value of Wad is -4.68 KJ, applying this value to the expression ln(pi/pf) should yield a positive result of 0.750 instead of -0.750, correct? This is due to the minus sign in the equation.
-
Gas Pressure
I realized that the mistake I made was a technical one, and not related to the issue at hand. My assumption of using Newton's second law would only make sense if the pressure unit was in Pa or N/m^2. However, since the pressure value was given in lbf/in2, the value obtained by multiplying the area with P (in lbf/in2) is numerically equal to the mass value. The response was a bit unclear, but it should have been understandable. As you pointed out, the weight of one pound is equivalent to one lbf here on earth. Therefore, if they wanted to clarify this, they could have done a conversion using the conversion factor of 1. However, this conversion should be understood even if it is not explicitly mentioned.
- Gas Pressure
-
Gas Pressure
My colleague said that if you know how many square inches there are, you can determine how many pounds are up there based on how many pounds are in each square inch, and it can be obtained by multiplying: area*p But again, for me, the value obtained is in force unit and not in massa unit. What am I not understanding?
- Gas Pressure
-
Gas Pressure
Guys, why in this question did the question answer only perform the product between area*P to calculate the mass? Question: Answer: Shouldn't the mass be calculated as m = (P*A)/g? Considering that: P = F/A, since: F = m*g P = (m*g)/A P*A = (m*g) m = (P*A)/g By performing dimensional analysis, you can indeed arrive at the value and unit: 2900 lb. However, I don't understand why gravity wasn't considered.
-
Quantization of angular momementum
-
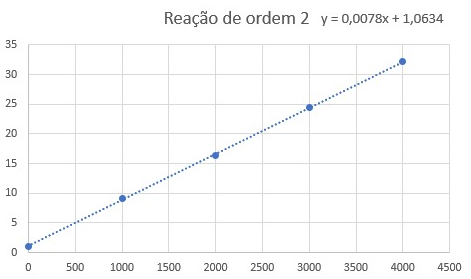
Integrated Rate Laws
I have a question about the value obtained by Rate Law. For example, in this case: the value of rate obtained by this equation is the global rate of reaction, right, and not the rate of a specific specie, I know that using this global rate I can find the species rate, multiplying for his stoichiometric numbers. Is my assumption correct? Because if the answer is YES, this question no make sense for me. I was trying to solve this question: I created this graph (Title is in Portuguese): I used "1/[HI] x t," which indicates a second-order reaction with a rate law equal V = k[HI]^2, so, the slope of the graph is numeric equal for k, AND, if my previous assumption is correct, the value obtained for V is the global rate reaction, as a consequence, the constant (k) 0.0078 (L.mol-1s-1) represent the unique rate law and not of specific reactant (HI). However, the gab, says that 0.0078 represents the rate constant (K) for the HI For me, it makes no sense, because, if it is correct, the value of V obtained by rate laws is the rate of HI and not for the global reaction since k refers to him and not for the global reaction.
-
What does "The calculation has reached convergence" mean in Quantum ESPRESSO?
Thanks. Thank so much,
-
What does "The calculation has reached convergence" mean in Quantum ESPRESSO?
I am a beginner in QE and I have a question. During I was reading about Output file, I saw a part where have it: "The calculation goes on step by step until convergence is reached. Then we show all the energies of all bands (eigenvalues of the Kohn-Shan orbitals) for each k-point, the Fermi energy, the total energy, the contributions of all the terms to the total energy, and the calculation time used by each subroutine of the program." Output file: ... iteration # 9 ecut= 100.00 Ry beta=0.70 Davidson diagonalization with overlap ethr = 1.00E-13, avg # of iterations = 1.0 negative rho (up, down): 1.151E-06 0.000E+00 total cpu time spent up to now is 28.4 secs End of self-consistent calculation k = 0.0000 0.0000 0.0000 ( 3909 PWs) bands (ev): -17.8569 -5.9595 -1.3957 -1.3957 4.4242 9.2594 9.9561 9.9561 k = 0.0000 0.0550 0.0000 ( 3909 PWs) bands (ev): -17.8034 -5.8955 -1.6155 -1.5115 4.4968 9.3266 9.9837 10.1886 ... -10.9411 -10.9411 -8.9101 1.6562 1.6562 12.4084 14.2298 14.2298 the Fermi energy is 1.6562 ev ! total energy = -22.80493136 Ry Harris-Foulkes estimate = -22.80493136 Ry estimated scf accuracy < 1.5E-15 Ry The total energy is the sum of the following terms: one-electron contribution = -44.02527202 Ry hartree contribution = 24.36506286 Ry xc contribution = -6.99363523 Ry ewald contribution = 3.85762303 Ry Dispersion Correction = -0.00870488 Ry smearing contrib. (-TS) = -0.00000512 Ry convergence has been achieved in 9 iterations Writing output data file grafeno.save init_run : 2.26s CPU 2.29s WALL ( 1 calls) electrons : 20.10s CPU 20.24s WALL ( 1 calls) ... Parallel routines fft_scatter : 4.35s CPU 4.44s WALL ( 23020 calls) PWSCF : 28.46s CPU 28.70s WALL This run was terminated on: 11:51: 0 5Sep2017 =------------------------------------------------------------------------------= JOB DONE. =------------------------------------------------------------------------------=
-
Enthalpy and Internal energy
So, is the true value for work +22, because work is done on the system? I used the 𐤃U = q+w. because I studied in a chemical class. However, I considered the contribution to work as negative. The book on the other hand, used a positive signal for work. The images is from the book.
-
Enthalpy and Internal energy
I have a question about the resolution of this exercise: I tried to solve this problem with the following steps: For the 1º law of thermodynamics, 𐤃U = q+w. As q = 𐤃H at constant pressure, I assumed that in this process q = - 15 kJ. As a result, 𐤃U = -15 - 22 (because was an expansion) and for this reason, 𐤃U = -37 kJ. However, the book has this resolution I understood why the book used this method, but I did not understand why my method is wrong.
-
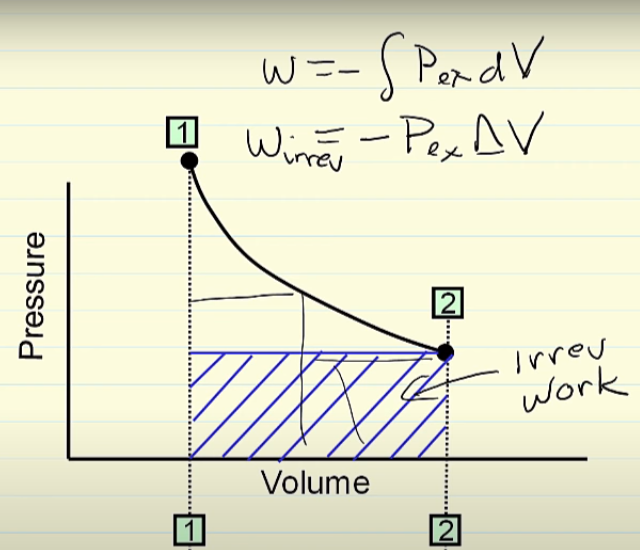
Why in an irreversible expasion, the pressure is constant
Why in an irreversible process, is the pressure constant? I understood why I should use Wirrev = -PΔV, but I did not understand why the pressure is constant in this process.
-
Nucleophilicity