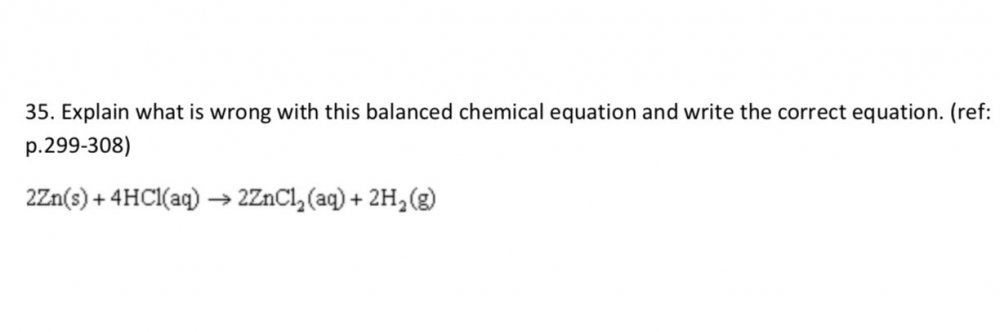Rachel Maddiee
Senior Members-
Posts
108 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by Rachel Maddiee
-
How would I show the rules that were applied for naming cations and anions that helped me to determine the name?
-
Yes, I’m aware. I couldn’t find anything in my reference book so I was trying to find a better explanation online. Phosphorus pentachloride (PCI5) is an exception to the octet rule because it has more than 8 electrons around the valence shell. Phosphorus (PCI5) can form an expanded octet containing ten electrons. The central phosphorus atom is bonded to five chlorine atoms. While PCI3 contains a phosphorus atom with an octet. How is this?
-
n order for the P atom to form bonds with 5 Cl atoms, it must have an expanded octet.
-
Phosphorus pentachloride (PCI5) is an exception to the octet rule because it has more than 8 electrons around the valence shell. The chlorine atoms (PCI3) obey the octet rule because the atom has five electrons and need three to fulfill its octet, while the phosphorus atom does not. Phosphorus (PCI5) can form an expanded octet containing ten electrons in the 3d orbitals. is this correct?
-
Transition metals Fe2+ has the lower state than oxygen (Fe3+ has the higher state), so “Fe” becomes ferrous. The name of Fe2+O can also be written as ferrous oxide. The terms “ferric” and “ferrous” are both use to refer to ions containing iron, since iron’s symbol is “Fe.” Ferrous = iron(II) Ferric = iron(III) Oxide = O-2 Anions always take the –ide suffix. Can i leave this?
-
Equation is not simplified. It should be Zn + 2HCl —> ZnCl2 + H2 How do do I explain what is wrong with this equation?
-
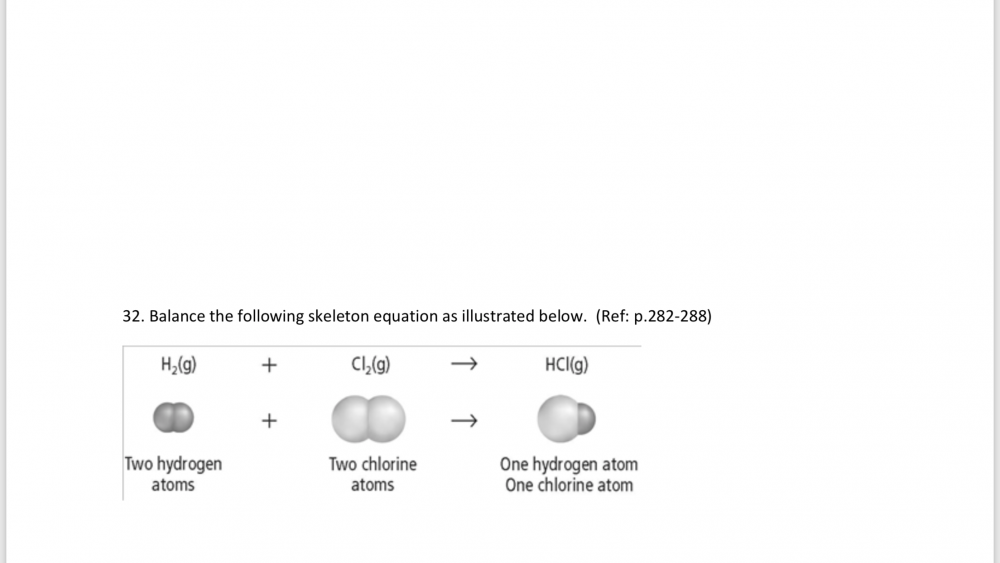
I have to show my work for this question and I’m not sure if this is the correct way to do it H2(g) + CI2(g) —> 2HCI(g) The balanced equation is H2 + CI2 —> 2HCI There are two hydrogen (H) atoms on the reactants side and two chlorine (CI) atoms on the reactants side. Two hydrogen (H) atoms and two chlorine (CI) atoms are produced. To balance them, we put a coefficient of 2 in front of the product hydrogen chloride (HCI).
-
I don’t really know how to do the calculations (for showing my work.)
-
Okay got it, but is there a better way that I can show how the name is determined?
-
I’m suppose to give how I determined the name though..
-
So how should I write it then?
-
I looked this up. I’m suppose to explain how I determined that is the correct name but I was struggling trying to come up with an explanation. Ferrous = iron(II) Ferric = iron(III) Here is the example in my book.
-
Yes, this is part of my studies. I’m following the example in my book but I need to show my work along with it so I was having trouble with that. I wasn’t sure if my explanation was correct.
-
Can you please give me a hint to what kind of explanation it should be? are you referring to electron configuration?
-
two Fe atoms have an oxidation state of +3? I’m not sure how else to explain it?
-
Okay, thank you so much!!
-
The Lewis structure is not correct. There should be two pairs of electrons (a double bond) between the C and O, and no lone pair on the C. Carbon needs 4 bonds and Oxygen 2. Carbon has 4 valence electrons and oxygen has 6 valence electrons. Carbon shares two electrons with the two Hydrogens, and shares four electrons with the Oxygen, (a double bond.) Correct structure: 2(1 for each H) + 4(for C) + 6(for O) = 12 valence electrons Incorrect structure: 8 bonding electrons and 2 lone pairs Number of electrons = 6 + 8 = 14 total electrons The Lewis structure is incorrect because it has too many electrons (to get to 12 electrons, we have to put in a double bond)
-
The iron ion Fe3+, for example, has an oxidation number of +3 because it can acquire three electrons to form a chemical bond, while the oxygen ion O2− has an oxidation number of −2 because it can donate two electrons.
-
The formula represents one molecule of ferric oxide, consisting of 2 atoms of iron and 3 atoms of oxygen.
-
So why does the question ask to explain which rule the structure does not obey? Since it satisfies the octet rule. Hw would I write it?
-
It reels me that there are too many electrons in the structure but I’m still struggling with the specific rule.
-
So I should definitely include lightwave energy?
-
I think I’ll stick with “wave” since that’s how it defined in my book
-
But photon is not in my reference page..
-
The Lewis structure is not correct. There should be two pairs of electrons (a double bond) between the C and O, and no lone pair on the C. Carbon needs 4 bonds and Oxygen 2. Carbon has 4 valence electrons and oxygen has 6 valence electrons. Carbon shares two electrons with the two Hydrogens, and shares four electrons with the Oxygen, IOW, a double bond. 2(1 for each H) + 4(for C) + 6(for O) = 12 valence electrons Carbon has formed three covalent bonds in the structure so it would have 7 electrons around it (not 8 as shown) Likewise the oxygen fails to follow the rule. This is what I have so far but I’m still working on the rule to finish it.