

John Ye
-
Posts
83 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by John Ye
-
-
Conflict.
Coulomb law needs electron to have certain and exact position, quantum theory tells that exact position is unknown.
This is the logic.
1 hour ago, John Ye said:Studiot,
If I incorrectly quoted your words, I say sorry to you. The text structure is not very clear to me, I will pay much attention to avoid the error.
According to standard quantum textbook, you're right. But unfortunately, the real cause of magnetism have not been totally clear to mankind. For example, in quantum textbook, the explanation of paramagnetism and diamagnetism are not sure. There are some exceptions.
I know electric current, and know magnetism. Besides textbook, I used to have had some thinkings of mine.
Conflict.
Coulomb law needs electron to have certain and exact position, quantum theory tells that exact position is unknown.
This is the logic.
1 hour ago, John Ye said:Studiot,
If I incorrectly quoted your words, I say sorry to you. The text structure is not very clear to me, I will pay much attention to avoid the error.
According to standard quantum textbook, you're right. But unfortunately, the real cause of magnetism have not been totally clear to mankind. For example, in quantum textbook, the explanation of paramagnetism and diamagnetism are not sure. There are some exceptions.
I know electric current, and know magnetism. Besides textbook, I used to have had some thinkings of mine.
that potential is Coulomb law's vibrant.
an integration or a differential between them.
exact position (at least radial) is needed.
0 -
3 minutes ago, studiot said:
Your model involves a one proton-one electron system.
As such the one electron must, by definition be unpaired.
Any unpaired electron has a magnetic moment.
So, by definition your system involves magnetism.
I have already told you why, by definition, even one moving electron constitutes an electric current.
PLEASE DO NOT PUT WORDS I DID NOT SAY INTO MY MOUTH
Studiot,
If I incorrectly quoted your words, I say sorry to you. The text structure is not very clear to me, I will pay much attention to avoid the error.
37 minutes ago, studiot said:Your model involves a one proton-one electron system.
As such the one electron must, by definition be unpaired.
Any unpaired electron has a magnetic moment.
So, by definition your system involves magnetism.
I have already told you why, by definition, even one moving electron constitutes an electric current.
PLEASE DO NOT PUT WORDS I DID NOT SAY INTO MY MOUTH
According to standard quantum textbook, you're right. But unfortunately, the real cause of magnetism have not been totally clear to mankind. For example, in quantum textbook, the explanation of paramagnetism and diamagnetism are not sure. There are some exceptions.
I know electric current, and know magnetism. Besides textbook, I used to have had some thinkings of mine.
2 hours ago, swansont said:It does not fail. Quantum theory does not modify Coulombs law. What is modified is the notion that we can know positions exactly.
You might not that Coulomb's law itself does not appear in the Schrödinger equation. It is cast as a potential, not as a force, and at no point are you needing to know where the particle is at any point in time, only that the electron experiences this potential due to the charge on the proton, no matter what its actual position is.
It would be good to show evidence which supports this. Like from scattering experiments, which will depend on the spatial details of the interaction. Can you predict what charged particle scattering should look like with your potential?
The ignorance here is staggering.
Are you aware of how our moon formed? The asteroid belt?
If something added as much relative energy to our solar system as is added in a discharge tube, our siolar system would be greatly modified.
Quantum theory explains this, but you don't seem very interested in actually learning these details. You have apparently already decided quantum theory is wrong.
0 -
33 minutes ago, studiot said:
As before you don't know what you are talking about.
Earnshaw's theorem does not depend upon Coulomb's Law.
Coulomb's Law is a sufficent condition because it obeys Laplace's equation.
But it is not a necessary condition because many other functions also obey Laplace's equation.
So it is very big hearted of you to allow Earnshaw with all the benefit of 21st century knowledge.
You have absolutely no idea what an electric current is.
And you appear to be denying the observed magnetic effect of moving charge.
Are you invoking God for this as well?
You have explained nothing, either in principle or mathematically.
Yet you have the affrontery to challenge professional spectroscopists to calculate spectra mathematically, and to mock the very real use our modern ability to observe and measure such spectra has brought to modern medicine and its ability in turn to combat plagues.
Blame God for the plagues and praise modern medicine for curing them.
Because you can't do it, here is a mathematical comparison of the formulae for the kinetic energy of an isolated particle confined to a rectangular box of dimensions a x b x c.
Clasically the energy is given by
E=Mv22In Quantum Mechanics the energy is given by integer values of the parameter n which approach infinity in number.
E=h28M[(nxa)2+(nyb)2+(nzc)2+]Can you see why we normally choose the classical equation, which yields sensibly the same results?
These formulae are given in mathmarkup language (MathML) or LaTex.
There are tutorials on this site about this.
You may need to refresh your page for the system to automatically translate the code into maths.
As a matter of interest can you maths tell you if the classical energy obeys Laplace's equation?
You have absolutely no idea what an electric current is.
And you appear to be denying the observed magnetic effect of moving charge.
Are you invoking God for this as well?
-------
My model involves neither electric current no magnetic things, which I think you know for sure.
The word God is not proper here, I just want to emphasize my previous words.
My model is very very simple, it just extends the basic Coulomb law, based on formula 2, and using the basic integration, got the energy levels and the spectrum.
It's the simplest math.
So I don't need to use Laplace equation and etc. I know little about that.
15 minutes ago, John Cuthber said:Based on observation, it isn't.
You can have diamagnetic materials which allow you to set up stable repulsion.
The interesting thing is that diamagnetism only works because the electrons are in continuous motion.
This proves your idea wrong.
You can stop wasting time on it now.
You can make a computer simulation, see if the system can be stable or not.
Use the clasical Coulomb law, simulate atom Li
I don't think it can be stable if the electron is stationary
0 -
8 minutes ago, John Cuthber said:
That's nice for you.
Are you aware that Earnshaw's theorem (as originally written) is wrong (or doesn't apply to the real world)?
https://en.wikipedia.org/wiki/Magnetic_levitation#Diamagnetic_levitation
Based on Coulomb law, Earnshaw's theorem is correct.
In fact, this does not necessarily need a theory, We can imagine its conclusion. The electrons can be stay in a stationary position because of the attractive force.
0 -
17 minutes ago, swansont said:
It does not fail. Quantum theory does not modify Coulombs law. What is modified is the notion that we can know positions exactly.
You might not that Coulomb's law itself does not appear in the Schrödinger equation. It is cast as a potential, not as a force, and at no point are you needing to know where the particle is at any point in time, only that the electron experiences this potential due to the charge on the proton, no matter what its actual position is.
It would be good to show evidence which supports this. Like from scattering experiments, which will depend on the spatial details of the interaction. Can you predict what charged particle scattering should look like with your potential?
The ignorance here is staggering.
Are you aware of how our moon formed? The asteroid belt?
If something added as much relative energy to our solar system as is added in a discharge tube, our siolar system would be greatly modified.
Quantum theory explains this, but you don't seem very interested in actually learning these details. You have apparently already decided quantum theory is wrong.
Does Coulomb law violate quantum theory? It tells us certain thing about electron--the force is certain with certain distance given?
0 -
20 minutes ago, studiot said:
By the way the planetary/satellite model of the atom is due to Rutherford, not Bohr.
https://www.britannica.com/science/Rutherford-atomic-model
Rutherford introduced his model as a result of experimental evidence, thus moving on from Thompson's earlier 'plum pudding' model.
Bohr introduced a modification of great significance, following De Broglie.
Sommerfield made what was probably the last major improvement to this semi classical model by introducing elliptical orbits.
So yes, the atomic model we are discussing here is the Bohr model.
And yes the Scientific method has been shown by this process to allow for the possibility of continual improvement.
On the other hand you have not addressed my comments, whilst continuing to spout nonsense, so I am reporting this and asking for this thread to be closed.
If you wish to discuss God, I'm out of here.
I am reading your paragraph
15 minutes ago, John Ye said:I am reading your paragraph
Studiot,
I have read Earnshaw's theorem segment.
based on Coulomb law, Earnshaw's theorem is right. But you should remember I extended Coulomb law. Actually, Coulomb law is wrong indide atom size world.
I will read the remaining part of your text., And give you feedback.
Now this segment:
Here we don't deal with electric current. It flows in conduct wire. We bypass it
32 minutes ago, John Ye said:I am reading your paragraph
Studiot,
I have read Earnshaw's theorem segment.
based on Coulomb law, Earnshaw's theorem is right. But you should remember I extended Coulomb law. Actually, Coulomb law is wrong indide atom size world.
I will read the remaining part of your text., And give you feedback.
Now this segment:
Here we don't deal with electric current. It flows in conduct wire. We bypass it
The following segment explain no_crash by magnetic field. It's unnecessary in my model. It's a patch. Without using any magnetic things, electron doesn't crash because of balanced point.
By stationary I mean the basic and commonsense word meaning, which we needn't extra explanation.
Next segment is about the curves, attractive far repulsive near, I already answered.
0 -
9 minutes ago, John Cuthber said:
Yes, the big difference it makes is that your model is plainly wrong because it violates the uncertainty principle.
(In fairness, I should point out that Bohr's model violates classical electromagnetism, which is why it's no longer in use.)
Does Coulomb law violate electron's uncertainty? It tells us certain thing----the force is certain with certain distance given?
0 -
22 minutes ago, Strange said:
Nonsense
This is fact. Can you calculate helium's spectrum by each of them?
35 minutes ago, John Ye said:Both Bohr model and quantum model are only working for H atom, one electron and one proton. My model works for H, and partially for helium, even Li, as long as they have equal opportunity electrons configuration.
Now I am telling why satellite model is joke. This is only one of many flaws.
Bohr electron's track radius is proportional to nn, where n is energy level number.
If n=7, 49R, electron will be running almost at MOON. It is a big joke, isn't it?
Another joke is this:
Solar system is a permanent system, once it shaped, it will never be dismantled and rebuilt until it vanish.
How about hydrogen atom?
It keeps bing dismantled and then rebuilt, right? In discharge tub, even in sky air, everywhere.
How can it be rebuilt without crashed electron? There must be a God to help out.
If dismantled Mars or Earth were rebuilt, they would crash into sun with the most probability.
A ridiculous joke.
0 -
54 minutes ago, studiot said:
Of course it is not accidental both your analysis and the original Rutherford-Bohr analysis are empirical in that theory are adjusted to fit the same experimental observations.
What I am telling you is that your analysis is well over 50 years old.
Do you contend that we have learned nothing in the last 3/4 of a century?
I remember in the 1960s this idea of attraction afar and repulsion at close range was put forward at basic physics level.
But it is an overall effect, a combination of many things.
You are incorrectly ascribing it to one cause.
It is particularly because of this I have been trying, unsuccessfully, to get you to discuss the mechanism of your 'balance point'.
You have got this fundamentally wrong because you have completely missed something out here.
That is why your analysis does not work for any system more complicated than a one proton-one electron system.This thread has 100+ posts and all wasted because you have made a fundamental error right at the beginning.
So where do the particles obtain this thermal energy to have a higher temperature?
I see no mechanism available in your proposed system.
Fine I await your comments.
Both Bohr model and quantum model are only working for H atom, one electron and one proton. My model works for H, and partially for helium, even Li, as long as they have equal opportunity electrons configuration.
Now I am telling why satellite model is joke. This is only one of many flaws.
Bohr electron's track radius is proportional to nn, where n is energy level number.
If n=7, 49R, electron will be running almost at MOON. It is a big joke, isn't it?
-1 -
5 minutes ago, Strange said:
True. Yours is very limited. And has even more problems than the Bohr model.
Satellite model is completely a joke.
People's mind was mislead by sun and earth system.
You may calculate the speed of Fe's inner layer electrons with satellite model, see what happens.
45 minutes ago, studiot said:Of course it is not accidental both your analysis and the original Rutherford-Bohr analysis are empirical in that theory are adjusted to fit the same experimental observations.
What I am telling you is that your analysis is well over 50 years old.
Do you contend that we have learned nothing in the last 3/4 of a century?
I remember in the 1960s this idea of attraction afar and repulsion at close range was put forward at basic physics level.
But it is an overall effect, a combination of many things.
You are incorrectly ascribing it to one cause.
It is particularly because of this I have been trying, unsuccessfully, to get you to discuss the mechanism of your 'balance point'.
You have got this fundamentally wrong because you have completely missed something out here.
That is why your analysis does not work for any system more complicated than a one proton-one electron system.This thread has 100+ posts and all wasted because you have made a fundamental error right at the beginning.
So where do the particles obtain this thermal energy to have a higher temperature?
I see no mechanism available in your proposed system.
Fine I await your comments.
By the way, how to read that special ASCII char formatted text? What tool to use? Thanks.
0 -
16 minutes ago, studiot said:
Of course it is not accidental both your analysis and the original Rutherford-Bohr analysis are empirical in that theory are adjusted to fit the same experimental observations.
What I am telling you is that your analysis is well over 50 years old.
Do you contend that we have learned nothing in the last 3/4 of a century?
I remember in the 1960s this idea of attraction afar and repulsion at close range was put forward at basic physics level.
But it is an overall effect, a combination of many things.
You are incorrectly ascribing it to one cause.
It is particularly because of this I have been trying, unsuccessfully, to get you to discuss the mechanism of your 'balance point'.
You have got this fundamentally wrong because you have completely missed something out here.
That is why your analysis does not work for any system more complicated than a one proton-one electron system.This thread has 100+ posts and all wasted because you have made a fundamental error right at the beginning.
So where do the particles obtain this thermal energy to have a higher temperature?
I see no mechanism available in your proposed system.
Fine I await your comments.
Thanks. I need time to learn and think it.
0 -
14 minutes ago, Strange said:
You agree it works then? And works better than your modified Bohr model.
Bohr electron must be spinning, mine doesn't. They are absolutely different.
Not all working models are created equal.
Whose is simple? Whose are presenting physical nature?
The geocentric theory had been working for centuries, but it is wrong.
0 -
40 minutes ago, Strange said:
Your modification of Bohr’s model doesn’t make much difference. It still doesn’t work.
Your silly straw man attacks on quantum theory do nothing to support your idea.
I know what quantum theory means, and what its result means.
I am not attacking it, just saying the physical fact. I haven't thought of that this could make some believer feel unhappy. I am sorry for my too straightforward words.
0 -
15 hours ago, John Ye said:
Yes, my work doesn't deal with fine structure things. It only works on basic spectrum.
John,
What? I rehashed Bohr's model? Bohr electron must keep running, while in this model electron is not necessarily running, it can be totally stationary.
This makes big and obvious difference, you didn't see, did you?
0 -
8 hours ago, John Cuthber said:
You would have been nearer to making a point if you had cited the scanning tunneling electron microscope.
But you would still have been wrong.
The uncertainty principle wins.
Because it gives the wrong answer.
Pretty much the whole of classical physics fails at this scale.
Learn to live with that fact.
John,
Does Coulomb law fails with quantum scale? It should belong classical physics, because it tell us certain thing----the force is certain with certain distance given
-1 -
5 hours ago, studiot said:
I see that you are still not listening.
Pity for you.
I will try one last time to lay out the logic as to where you are right (yes in some places you are indeed right) and where you are just plain wrong.
First of all, classical electrostatics forbids you to have a static system of electric charges, under coulomb forces alone.
In particular Earnshaw's Theorem say
OK so if we have a system of two or more charges, the charges must be moving. Period.
In this case the proton is approximately 1800 times as massive as the elctron so we take the proton reference frame as the basis and refer the electron's motion to it.
So the electron is moving relative to the proton.
Now an electron in motion is the definition of an electric current.
And an electric current has an associated magnetic field.
So there is an associated magnetic field, hence the Biot Savart Law is applicable.
Note by 'stationary' Wiki means steady.
So you have said that the electron would crash into the proton under coulomb forces.
Why?
For the same reason the Earth does not crash into the Sun under classical gravitational forces.
Because it is in motion.
So gravitational attraction provides the centripetal force to accelerate the Earth's trajectory into the path of a closed curve.
Similarly the coulombic attraction accelerates the electron's trajectory into the path of a closed curve.
That is essentially Bohr's satellite theory, as you have called it.
However the problem (acknowledged by Bohr and his contempories) is that an accelerating charge must interfere with its own magnetic field (Biot Savart or Lorentz) to generate electromagnetic waves.
But the electron in an atom does not do that. An electron in a cathode ray definitely does emit EM radiation.
There is no classical explanation for this.
The why is where the Quantum Theory enters but I will not pursue that here and now, since this is a completely classical analysis (like yours).
Now you have taken empirical measurements and calculated (with your proposal) the simple hydrogen first spectra, as Bohr did, and got pretty good agreement with observation, as Bohr did.
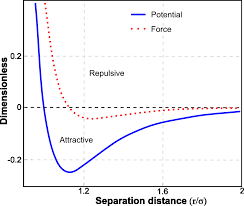
Does this graph look familiar?
It is the Lennard Jones Potential I mentioned earlier.
And it is very similar to your proposal, although the formula is more complicated.
This is also empirical.
Finally I asked you to look at one more thing.
The Madelung constan.
This is a method of calculating the combined effect of all other ( than its associated proton) positive charges influencing the electron on the other side to provide what you call your point of balance - the value you admit you can't calculate for yourself.
Now tell me again that these four pieces of Physics I recommended are not relevant.
Studiot,
Thank you for providing these here, which may help me a lot. I will read them later, and answer same part of the paragraph.
It's not accidental for the graph to look familiar.
Microscopic world has a regular pattern or basic law, that is, things are attractive while they are apart far enough, are repulsive while closer enough, and there must be a balanced point.
Electron and proton are the case. So do atoms (metal atoms build crystal), so do molecules. If they seem not attractive each other, it's because temperature is not low enough.
I will read and answer other part of your long text. Later
0 -
10 minutes ago, John Cuthber said:
You would have been nearer to making a point if you had cited the scanning tunneling electron microscope.
But you would still have been wrong.
The uncertainty principle wins.
Because it gives the wrong answer.
Pretty much the whole of classical physics fails at this scale.
Learn to live with that fact.
You use Coulomb law to solve equation,
You deny Coulomb law , claim it inapplicable after equation was solved.
You changed mind in less than one minute.
Is this behavior logical? And OK?
This is what quantum model does.
-1 -
6 minutes ago, swansont said:
No. Coulomb's law doesn't address this in any way.
Yes.
Yes.
Nobody is saying Coulomb's law doesn't exist, or doesn't apply.
You may recall how to solve H atom's Schrodinger equation.
Coulomb's law is used and applicable in the beginning of the equation solving process, Am I correct?
Why do you say it become not applicable after the equation was solved?
0 -
6 minutes ago, John Ye said:
in old TV's display tube, electrons has certainty. Or else, we would not be able to watch TV program.
In synchrotron, electrons have certainty.
Previously certain.
Coulomb's law tells us that an electron near a proton has certainty. right?
And to get H atom's spectrum solution, Schrodinger's equation uses Coulomb's law, right?
Then quantum solution says electron is uncertain.
We used A, we get B by A, then we said A does not exist.
12 minutes ago, swansont said:Which must have an electric dipole (or other mulitpoles, depending on how many electrons you have), which has never been measured, even though it should be simple to do.
Not so much that Coulomb lost (the Coulomb potential is still part of the QM model) as we discovered that the electron is also a wave, and that we have quantized energy levels, and all of this needs to be considered when discussing atomic structure.
Any purely classical approach will have things that are wrong, and things that are missing; examples of both have been shown in this thread.
Just because the uncertainty is small compared to the required precision does not mean there is no uncertainty.
In electron's double slit experiment, what is the distance between 2 slit?
and what is the size of a single pixel in the display tube screen?
How much do they differ?
48 minutes ago, John Cuthber said:Please show the experiments that tell us how ghosts move.
Like I said, you are so wrong, it's funny.
...you have broken the uncertainty principle.
And according to the uncertainty principle, it can't.
So, it doesn't.
We have two "laws" which give contradictory results. The way to find out which gets broken is to do the experiment.
We did.
Coulomb "lost".
Not many of those things were as ridiculous as your idea.
Can you use quantum model to calculate the Helium spectrum?
0 -
5 minutes ago, John Cuthber said:
Please show the experiments that tell us how ghosts move.
Like I said, you are so wrong, it's funny.
...you have broken the uncertainty principle.
And according to the uncertainty principle, it can't.
So, it doesn't.
We have two "laws" which give contradictory results. The way to find out which gets broken is to do the experiment.
We did.
Coulomb "lost".
Not many of those things were as ridiculous as your idea.
in old TV's display tube, electrons has certainty. Or else, we would not be able to watch TV program.
In synchrotron, electrons have certainty.
Previously certain.
0 -
5 minutes ago, Strange said:
Do you realise you quoted yourself and then said that?
You are the only one who seems to be arguing on the basis of "belief".
Meanwhile, scientists create models and set them. If the models work, they use them. Your model doesn't work, as you admit, therefore it is useless however much you like it.
I know what you said. It's hard to change people's mind. I am just playing for fun.
0 -
26 minutes ago, John Ye said:
Totally different models.
Electron must move, or electron can be stationary.
When you have one proton in left hand and one electron in right hand, and you put them on an absolute smooth table.
Electron will be attracted by proton and will be running toward proton.
What is the electron's moving line? is it a straight line. right? according to Coulomb's law, it's a straight line.
Can you imagine at what distance, does electron become a quantum ghost?
Or can you imagine at what distance, does electron start to change the straight line into a circle?
Why is it not crash into proton? According Coulomb's law, it must be crashing into proton. how to explain these?
We have been believing in some ridiculous things, for so long time.
0 -
20 minutes ago, John Ye said:
It's not a tweak of Bohr model.
Bohr model is a satellite model, electron MUST be circling the proton,
Quantum model is the same. Electron must be moving according to the probability outside proton. Because it moves like a ghost, we can call quantum model "ghost model".
In both models, electron must move. If it stopped, atom would vanish because electron would crash into proton.
In my model, electron does NOT need to move. It can be stationary.
In fact, if the temperature is low enough ( near absolute 0K ), all electrons in any atom will stop moving.
In normal temperature, my model's electron has only random thermal movement.
So I called the model "static electron configuration model"
Totally different models.
Electron must move, or electron can be stationary.
17 minutes ago, Strange said:You can call it what you like. It still works.
This is not true of the quantum model. You don't appear to know what you are taking about.
But, as you have said, your model is no more useful that the Bohr model.
When you have one proton in left hand and one electron in right hand, and you put them on an absolute smooth table.
Electron will be attracted by proton and will be running toward proton.
What is the electron's moving line? is it a straight line. right? according to Coulomb's law, it's a straight line.
Can you imagine at what distance, does electron become a quantum ghost?
Or can you imagine at what distance, does electron start to change the straight line into a circle?
Why is it not crash into proton? According Coulomb's law, it must be crashing into proton. how to explain these?
0 -
3 hours ago, Strange said:
And it only works with hydrogen (and partly with helium). Not very useful then is it. It looks like your attempt to tweak the Bohr model hasn't really worked.
Unlike, oh let's just say ... quantum theory.
It's not a tweak of Bohr model.
Bohr model is a satellite model, electron MUST be circling the proton,
Quantum model is the same. Electron must be moving according to the probability outside proton. Because it moves like a ghost, we can call quantum model "ghost model".
In both models, electron must move. If it stopped, atom would vanish because electron would crash into proton.
In my model, electron does NOT need to move. It can be stationary.
In fact, if the temperature is low enough ( near absolute 0K ), all electrons in any atom will stop moving.
In normal temperature, my model's electron has only random thermal movement.
So I called the model "static electron configuration model"
0



A new atom model (static electron configuration model )
in Speculations
Posted
John,
How to quote and reply part by part as what you did?
I will never change my mind.
The static electron configuration is the true picture of atom. no spinning, no ghostly jumping.
I know it's hard to be accepted today, for that quantum model have been existed so long time.
But I am sure there must be one day in the future.