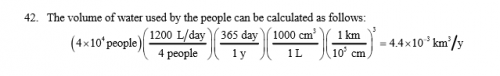esig
Members-
Posts
12 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by esig
-
Those are the answers given by my teachers. But I have calculates time of flight, although I do not get the exact same number as given by my teachers but they are close. For instance I get 2,2449 seconds the rock is in the air but the teacher gave 2,262 s And I get the distance as 23,3297 m, but I do get the same as the teacher if I use the same time he got witch is 23,51 m. Max height is above starting point. I am not sure how to figure out where the vertical velocity is zero. How is it simplified? Have figured it out. Thanks for your help
-
I have an exam tomorrow and I have spent 3 hours trying to figure this out. I have the answer but not the method. You throw a rock from an edge at the speed of 12 m/s at an angle of 25°. You are 1,5 m above the edge and it is 10 m above the ground. No air resistance. Have calculated max height and time 0,61 s and 3,3 m b) how long is the rock in the air? c) how far from the edge (horizontally) does the rock land? d) what speed does the rock have when it lands? Answers b) 2,3 s (2,262) c) 24 m (23,51) d) 19 m (19,22) Please help me, am a bit desperate, really want to pass this test! I have figured out b and c Still need help with d
-
I am sorry but english is not my first language, what do you mean by that I need to cube the conversion factor as well?
-
I have a problem and I have the answer and the method in the book but when in put it in the calculator or excel or google I get the wrong answer The problem is An average family f four uses roughly 1200 liters of water per day. One liter =1000cm3 How much depth would a lake lose per year if it uniformly covered an area of 50 square km and supplied a local town with a population of 40000 people? Consider only population uses and neglegt evaporation and so on So I started my calculation and according to the book I did them right but my answer is not right. 40000 people(1200 L/d/4 people)(365 days/1 yr)(1000cm3 /1 L)(1km/10^5 cm) My calculation= 43 800 000 Books= 4.4*10^-3 I maybe thought that mine was in cm since the book gives it answer in km but if I did my conversion right I have 438 km and the books is 0.0044 km Can somebody tell what it is I am doing wrong? I took a screen shot of the books solution
-
I have an unknown sample of iron II I have the mass and I have done the titration with KMnO4. I mixed the sample with 100 mL of distilled water and then used 25 mL of that and addes 30 mL of distilled water, 30 mL of 3 M H2SO4 and 2 mL of 85% H3PO4. I need to find the balanced equation of the titration but I just can't seem to figure it out I understand that I first need to find the limiting reactant, but this is the one part of the entire report that I am having problems with and I just cant figure it out. Can somebody please point me in the right direction here? The mass of the iron is 2,2068 g if that matters
-
So once I find the limiting reactant that will tell me witch I should use? And to be clear I should use the limiting reactant in the equation?
-
yes I did mean that I have 20,0 g of 1 Molar solution of copper sulfate, and the Iron was powder and dissolved in the solution, the video was very interesting though. The answer is only supposed to be in kJ/mole nothing is mentioned of displacement. I have found delta t, balanced all the equations and found kj/mol for all the reactions exept for this one since.
-
I have two substances 20.0g 1M CuSO4 and 1g Fe. I have found the moles and have calculated this for other reactions but what I am not sure of is if weather i should use both substances for moles in the equation or just one, and if I should use just one then witch one? The equation in -q/1000/m I have 0,00002 moles CuSO4 and 0,018 moles Fe
-
I have studied balancing equations but I can never seem to get them right. Well not never, sometimes they come out right but about 90% of the time they come out wrong Good luck withe the chemical preparation
-
I took the measurements myself I have the formulas for the chemicals I used. NH4Cl, Na2CO3, NaHCO3, 2 M HCl, Fe (powder), 1 M CuSo4, 2 M CaCl2, 2 M NaOH, 2 M NaCO3, NaCl. Molar mass is basicaly just formula mass right, find the elements in the periodic table, find the atomic mass and that is the same as molar mass right? I did 5 reactions the first two were with distilled water and ammonium chloride and sodium carbonate. 3rd was the iron with the copper sulfate, the 4th sodium carbonate with calsium chloride and the last one was the effects of salt on ice. I know the chemichals react with each other but truthfully I am really bad at what is happening, I am supposed to write down in the 4th colum what chemical was made and the formula. IFor the first one I have H2O and NH4Cl and I am not sure how I am supposed to figure out the reactants (think that is the right word)
-
A bit similar but I am not making a graph. I am doing that for another part of the report though http://oi57.tinypic.com/35d0zra.jpg I included a print screen of the table, but it is in my language but then you get a better idea what I am trying to do
-
I am in way over my head here and a little help would be very appreciated. So I did an expiriment in school and am trying to write up the report but I am a little lost. I am supposed to set it all up in a table and calcutale the heat (sorry english is not my first language so I might not be clear on the correct chemistry terms). A few of theese would just be good to be clarified that I am doing the right thing, others I might a big nudge in the right direction. So I had a reaction with distilled water an ammonium chloride (one of 5 reactions we made) I am suppsed to put in the table the initial chemicals/ reaction chemical formula and molemass. next one is the amount of chemicals in g per moles. NExt is the final soulution and formula ( in this area i generally suck). next I have the beginning and final temperatures. Next is the change in temperature (I'm just supposed to do inital-final right? or am I supposed to do high-low?? very confused on that one). Then there is +- delta temperatur ( am I supposed to put in the uncertainty in the measument here?) The last two are the heat of the chemical reaction in kJ and heat per moles kJ/mole ( I think i have the kJ one figured out but not the kJ/mole one) I hope someone can help me before I go crazy