McCrunchy
Senior Members-
Posts
43 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by McCrunchy
-
Hello all, I 'm looking for a website/reference that would give the real and imaginary dieletric constants of various materials ( gases, water, solvents, oil, plastics, minerals, metals etc.) on a wide range of EM wavelengths. I would like to get an overview of who absorbs in what domain. Thanks for your help, McCrunchy
-
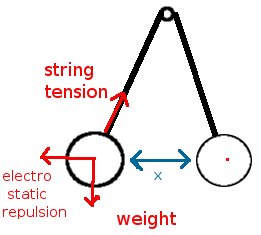
So I've found an easy method to get an approximate value for the charge on ballons, here it goes (see picture in attachment): - take two ballons, attach them with a thread of length l (in my case 80 cm) - anchor the center of the thread round an axis, a broom might do. The uncharged ballons hang from the thread touching each other. - rub each one of the balloons with wool or whatever material you are interested in. Be careful that the first balloon doesn't stick/discharge to your positive skin/sweater, etc. while charging the second one. - let the ballons loose, they're now at some equilibrium distance from each other (in my case ~5 cm) - measure the distance between them, deduce the angle alpha the thread makes with the vertical axis. - balance the three forces to deduce the formula : f_electrostatic = tan (alpha) * mass_ballon * g - get the charge from Coulomb's law. In my case no matter how hard I rubbed the ballons with my woolen sweater the distance between the balloons would be about 3-6 cm, which makes for about 10-7 C on each of them, or a 100 nC. Yours, McCrunchy
-
Hi all, I was wondering why is it that some coins need to be rubbed in order to be accepted by vending machines. Is it making the edge more conductive by removing some grease and thus allowing for a resistivity measurement inside the machine ? Any bright ideas, references ? Thanks in advance, McCrunchy
-
Hi, measuring the force would be possible, however you always need two balloons and you never really know whether they're identically charged (except for saying " hey , I rubbed it the same number of times with the same sweater, it must be equally charged !" . Plus it musn't be all that easy to measure. I thought of using a Faraday cup. Basically you collect all of the charge of the balloon on a initially neutral , isolated conductor, for example an aluminium cup, and measure the potential difference that results between the cup conductor and the ground. Whatever the shape of your cup, you have Q = C * V Q : charge - unknown C : capacitance, unknown, but constant V : voltage measured I'll try calibrating the device to figure out the C with a charged capacitor of known capacity ( I can't think of any other device that would provide a known amount of charge) McCrunch
-
Hi, does anyone have an idea of the amount of static charge (in Coulombs) that is transferred to a balloon ( 20 cm in diameter) upon rubbing it with wool ? I know it depends on all sorts of factors, how I rub, etc., but I'm just looking for an order of magnitude. Thanks, McCrunch
-
Ok, thanks to you all for the interesting answers, I'll postpone the patent procedure McCrunch
-
Hello all, I was looking at a corn field not long ago and thinking about the optimal shape of the field's surface to maximize yield. Why do we keeps fields flat ? If instead one were to shape them with hills and valleys, say as a sine wave, the net surface area would be much more than the surface of a flat landscape - that would make for more space to grow cucumbers, salad, carrots ... Of course the wavelength of the sine wave should be greater than the average size of the plants grown, so the vegetable themselves wouldn't "fill the holes". Although, the hills shouldn't be too steep so they wouldn't cast too big a shadow. What do yo think ? McCrunchy
-
Hi, What evolutionary advantage do leaf margin morphologies (dentate, crenate, siliate etc. cf http://en.wikipedia.org/wiki/Leaf) confer compared to a plain smooth edge ? My first idea would be that having more "edge" makes for swifter gas exchanges (a damp sheet of paper dries at the edges first). Thanks for your help, McCrunchy
-
Hello, I'm looking for a graph or values of the refraction index of water in the mid-IR region. Can anyone help ? McCrunchy
-
Light is diffused from tthe dry cloth because it is constituted of thousands of micrometer sized cellulose-type fiber, which has an index of refraction of about 1.3 (ie, different from that of air, which is 1). In order for something to diffuse light, you have two preconditions: - it should be rough on a micrometer length scale (about the wavelength of visible light) - it should have an index of refraction different from that of the surrounding media. What water does is : - it flattens the surface - the wet clothe's surface essentially becomes flat - it's refraction index matches that of the fiber (water : 1.33). So the cloth becomes transparent : indeed, if you look at a piece of wet cloth in transmitted light (that is, with the piece of cloth betwwen you and the light source), it let's through a lot of light ! Coming back to the dark wet spots, the transmitted light then bounces off your skin, losing in intensity and goes through the shirt again (losing more intensity): this is why it appears darker. The index of typical clothing fibers is best matched with oils: you've probably seen wax making paper transparent. McCrunch
-
Hello all, I have a slightly technical question. I'm trying to solve a problem on a stream (vertical jet) of hot water cooling in air (think of pooring tea). I want to compute the temperature of the water as it goes down. Now, since the water is accelerating (free fall), the speed of the jet is different in different places, and so is the heat loss. However, I do not know the dependence of heat loss on speed. Assuming the flow of air next to the stream is non-turbulent, I tried solving the heat equation, but with only one boundary condition (the stream/air interface), I spinned my wheels a bit and gave up. Is there a reference on this dependence ? It must be a classical pipe-type heat transfer problem. Thanks, Merged post follows: Consecutive posts mergedand disregarding the dependance of heat loss on speed, but rather taking the wikipedia value of still air's thermal coefficient, I get that a 30 cm long, 1 mm in radius stream of boiling water gets cooler by 1°C. I'd be curious to see by how much taking into account the speed of the jet would increase this value ... McCrunch
-
Hi Theo, Let me first of all clarify what you meant by stable, less stable : you can define this by the solubility product Ks of the precipitation reaction. The lower the Ks, the more the ions will be prone to turn into a crystal, the more stable the crystal. Typically, since I guess you're talikng about CaCO3, Ks-calcite < Ks-aragonite < Ks-vaterite By the way, Ks is directly related to Delta G CaptainPanic mentionned by Ks = exp(-Delta G/kT). Ostwald's kinetic law states that the first polymorphs to form from a supersaturated solution are the most soluble ones, ie, that would be vaterite first. The reason for this is that the ions find their way to a crystal structure in steps : they first form loose, highly soluble structures with water inside - ikaite for example for CaCO3. Gradually, the water molecules are "squished" out of the crystal, but this requires overcoming energy barriers, and this takes time. So thermodynamically the least soluble form is the most favoured one, but kinetically (Ostwald), its all reverse. The conclusions is that when kinetics are slow (low supersaturations), thermodynamics wins and you end up with calcite. On the contrary when you have high concentrations you typically get all sorts of polymorphs. To give you an example from my lab, mixing 20 mM NaHCO3 with 20 mM CaCL2 gives us a roughly a mixture half vaterite half calcite (determined from X-ray diffraction analysis). I don't know how well Ostwald's empirical law applies; but man, it's a law, so it must be based on more than a couple observations! Regarding your idea on seeding the solution with less stable polymorphs: I have indeed observed that seeds greatly reduce the induction time of precipitation reaction (ie, it accelerates the precipitation). This means that the ions accumulate on the seeds. Does this mean that they necessarily adopt the same polymorph ? Maybe. Only experiment can tell. McCrunchy
-
Hello, I was wondering, would it make any sense to try to get electricity from the IR part of the Sun's/Earth's radiation spectrum ? IR covers a very broad region of wavelengths, is emitted day and night, detectors are pretty cheap (they're the ones on your TV set) ... but there must be a reason people aren't really considering them. Thanks, McCrunch
-
It isn't exactly dilution, but you can add ethanol or acetone to a concentrated aqueuous solution of a salt to make it precipitate. Ethanol is miscible with water, but the salt's solubility decreases dramatically in the mixture. To get a list of lattice structures/growth habits for different minerals and powders, the best site I can recommend is : http://webmineral.com/ McCrunchy
-
Hello all, Picking up on Hermann Trude's idea (in a recent post that I was unable to find though) of watching atomic emission spectra of salt by burning them in ethanol/water solutions, I got the following results: - the pure ethanol/water flame was blue - CaCl2 gave characteristic red flashes - NaCl yielded an orange flame, not much different from the orange color of a candle's or matche's flame - neither KCl, nore BaF2 (which is insoluble in water by the way) yielded any perceptible changes from ethanol's blue flame. Do these results seem right to you ? Would you suggest any other salts ? I'd love to see some green, or pink, for example ... Thanks ! McCrunchy
-

Dissolve limestone without calcite damage?
McCrunchy replied to Mike Palescuk's topic in Organic Chemistry
Hum ... limestone is to a large extent calcium carbonate, so I think mechanically removing the limestone would be the better solution. -

Is color one wave of light or many waves together?
McCrunchy replied to seriously disabled's topic in Physics
http://mysite.verizon.net/vzeoacw1/coloradd.html McCrunchy -
Instead of saying "oh, there aren't any ways to prove that light are actually real waves, so God must exist", why don't you think a bit harder and find an experimental setup to prove that light IS actually a wave ? I'll give you a clue (and a bit of self-promotion on the way) : is a way to demonstrate that ultrasound is a wave.
-

How was the electromagnetic spectrum discovered?
McCrunchy replied to seriously disabled's topic in Physics
Hi, That's an interesting, but ambiguous question - I don't know whether "discovery" is the right term, but there are definitely periods in history where certain wavelength domains were investigated more intensely than others. For example, I heard Terahertz radiation generation (between 1 millimeter (high-frequency edge of the microwave band) and 100 micrometer (long-wavelength edge of far-infrared light)) was long neglected and is currently the object of intense research. The first question one has to address is when radiation was first recognized to be an oscillatory phenomena, ie, to which you can associate a frequency. This would be Young 1803 ( http://en.wikipedia.org/wiki/Double-slit_experiment ). This was the definite proof, but a lot of scientists had already gotten very familiar with this notion well before that : Huygens and his famous principle (1695, http://www.mathpages.com/home/kmath242/kmath242.htm ), Fresnel, etc. They assumed light travelled as a wave, however I don't who first discovered that red was lower frequency than blue. They all obviously understood why a prism decomposes light - the refractive index of a material depends on the colour of the impinging light -, but the relation between refractive index and frequency (see the graph in http://en.wikipedia.org/wiki/Refractive_index : refractive index decreases with increasing wavelength ) is not a thing one can easily deduce, I think, unless you know that light is an EM wave, and that came later with Maxwell ... Some other key milestones would be: - 1800, Herschel's discovery of IR radiation. Or let's rather say : he discovered some kind of invisible rays on the red side of the spectrum that heat stuff, see http://www.practicalphysics.org/go/Resources_16.html - ????, discovery of UVs ? I mean, someone must have discovered that you don't tan behind a glass window ! - 1864, Maxwell publishes his famous equation : light is electromagnetic radiation. - 1888, Hertz, antennas, which considerably boosted the discovery of "new" wavelength domains. From there on, "discovering" a new wavelength domain is more of a technological problem than anything else: it's equivalent to mastering the oscillation frequency of the current, at least for low-frequency to microwave type radiation. - 1895, Roentgen, X-Rays, citing Wikipedia: " He knew the cardboard covering prevented light from escaping, yet Röntgen observed that the invisible cathode rays caused a fluorescent effect on a small cardboard screen painted with barium platinocyanide" 10-20 years later Laue discovered that X-rays were none other than high frequency EM waves - 1900 , Paul Villard, gamma rays. "It wasn't until 1914 that Rutherford showed that they were a form of electromagnetic (EM) like light only with a much shorter wavelength than x rays." - 1903, N-Rays : that's a fun one, see http://en.wikipedia.org/wiki/N_ray - later : microwaves, cellphones - now : terahertz I hope this helps. I'm sure there are many more details and versions to be given of this history. I really do wonder when the picture of the radiation spectrum" as we know it ( http://en.wikipedia.org/wiki/File:Electromagnetic-Spectrum.png) first emerged in textbooks. It would be fun to compare what this picture looked like back in 1920, 1950s and now. Cheers, McCrunchy -
Hey, Have a look at my experiment on standing waves in a sonicator bath : McCrunchy
-
Hello, I had a look at graphs of average human height as a function of time , cf for example http://tpek2005.free.fr/Pages/Taille.htm . It seems that for most populations, height has been increasing almost monotonically for the past 2 centuries. I was wondering : will humans ever reach an optimum "plateau" height ? Or will they just keep growing ? There are lots of factors explaining why we should grow taller (attractiveness, health, etc.) or stop growing (heart has to do more effort to pump the blood etc.) ; more specifically, I am looking for a study of an animal's (any animal) size evolution - this would in my mind provide the best clues to answering this question. Thanks for your input, McCrunchy
-
There's a thing I don't get about the electron work function of a metal: in this wikipedia article, they say it's the difference between the bottom of the image-charge potential and the fermi level (just look at the picture in the article). But the image-charge potential has no bottom - it goes as 1/a, a being the distance between the electron and the metal surface . How do you get around that ? Does one have to reach for quantum mechanics to get a bottom - just as when solving for the hydrogen atom's energy levels ? If so, how did the likes of Einstein do to compute a work function before ~1915 ? Thanks in advance for your help, McCrunch
-

How can X-Rays ionize air? (or other molecules)
McCrunchy replied to mahela007's topic in Classical Physics
oops, indeed. Somehow I had imagined the electron-electron repulsion would make the ionization potential smaller than that of hydrogen ... but it's true we can almost neglect this repulsion to compute the energy levels [ and why is that again ? drifting from the initial subject ...] -

How can X-Rays ionize air? (or other molecules)
McCrunchy replied to mahela007's topic in Classical Physics
Just to give you some numbers, a photon in the X-ray domain has an energy typically around 8000 eV, whereas an electron's binding energy can be at most -13.6 eV, level 1s 's energy in hydrogen. So you have plenty of energy to pick it from the atom. This effect is put to practical use in X-ray gas detectors : they're filled with a neutral gas, typically xenon (which won't oxidize you're device once ionized), at a given pressure. When an X-ray goes through the gas chamber, it ionizes the gas. The released electrons are accelerated by an electric field to a cathode, where they read out as a small current. McCrunchy -
Swansont, I agree the black body we're heating also radiates heat out. At equilibrium, these fluxes equalize. This gives us : Qin=Qout, with Qout = A sigma T^4 . My argument is that as long as Qin can be made big enough by using a big enough lens to focus the rays, there's no reason why T couldn't reach an arbitrarily high value - even higher than that of the initial source of radiation. We don't need to be in the IR regime - let's consider a 3000 K emission source for which we know the lenses exist and work.