-
Posts
16 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by khatereh
-
-
Thank you for your reply
0 -
Could you please explain more?Do you mean this:
I should add basic aqueous solution of a sodium salt to it, Then dicarboxylic acid will form which is probably soluble in water. right? and diketone will remain?
Thanks for your reply by the way
0 -
-
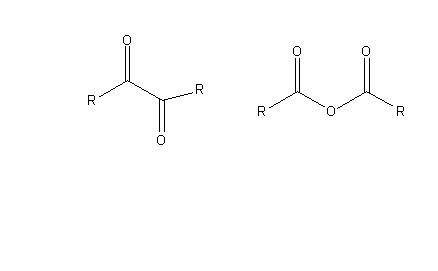
I have a mixture of an aromatic diketone and its anhydride. I was not able to separate them with column chromatography as they came out almost at the same time and the process was so time consuming in the case of using longer column. I also have to work with small amount of materials and most of my product was wasted in column..
I was wondering if anybody knows a better separation method?
0 -
So If you are not able to see any peak in carboxylic acid range of the HNMR , I think you should doubt that your compounds contains carboxylic acid group. see following IR spectrum

If your spectrum contains this kind of broad peaks at around 3300, and if it is this much small in comparison with other peaks. it is not a reliable peak to assign it as OH of carboxylic acid group.
you have to do other characterization to get the structure. at least from the reaction that you have done you should have an idea what you are expecting to see.
following websites can be helpful for characterization:
http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng
http://www.chem.wisc.edu/areas/reich/handouts/nmr-h/hdata.htm
http://www.chem.wisc.edu/areas/reich/handouts/nmr-c13/cdata.htm
0 -
The free hydroxyl group ( I mean without hydrogen bonding) normally is not very broad in IR. I agree with hypervalent_iodine, if you provide the spectrum it will be great.
Also I should note that for characterization of an unknown compound, one method is not enough. HNMR also can confirm the idea of having carboxylic acid group ( broad peak around 12ppm).
0 -
Sounds like an attenuated reflectance IR experiment?
They probably used "diamond anvil cell" for their FTIR which can be used when small amount of samples is provided (your sample will be usable easily after getting spectrum). I doubt that it is reflectance IR experiment.
0 -
Am I the only one who finds the question very ambiguous? Could you perhaps quote the exact question?
I want to find the error for each lnk.
 0
0 -
OK sure!!!
Small note: the definitions of what is a base and what is a nucleophile do differ, but you should have also elaborated on what you meant by that, as it is a little confusing. Nucleophiles are Lewis bases by definition. So, all nucleophiles are in fact bases, but not all bases are nucleophiles. The distinction that you often see is that a nucleophilicity is all about reaction rate, whereas bases are all about the stability of the end product and by association, reaction equilibrium.
A simpler explanation comes from considering the fact that oxygen is more electronegative than sulphur, which means that it is less likely to want to donate its electrons to attack an electrophile. Atomic size comes into play due to the fact that you get better orbital overlap and because the nature of the orbitals makes them more susceptible to being polarized, which increases nucleophilicity. This in turn makes reactions such as the SN2 reaction occur via a transition state that is lower in energy. There is also the hardness of the nucleophile to consider. This isn't really related to the OP, though.
The size of the molecule as a whole is more or less how you've described. I'm not going to elaborate, however, as SFN has a policy of not providing answers to homework questions. In future, if you could please provide less in the way of straight up answers to these sorts of questions and more in the way of guidance and help towards getting the answer, it would be appreciated. That way the student actually learns something.
0 -
How did you run the IR spectrum?have you made a disk with KBr ?or is it in Nojul mull? or have you made a solution?
I got a IR spectrum of my unknown compound and it shows a peak which is sharp(small in size) but not as strong as it should be for a carbonyl stretching at 1732. Also a small and bit broad peak at 3356 which confuses me the possibility of O-H stretching which is normally very broad. Please help me regarding this
0 -
Basity is different from nucleophilisity.
Bigger atoms are better nucleophile because they can polarize better than the small atoms because of that SH is better nucleophile than OH. but OH is better base because it is small and it can grab the protons more easier than SH.
Alcohols are not strong nuclephile. but if they want to act as nuclephile, the less bulky should be better for attacking to the electrophile.
if you mention the reaction that you are looking at, I might have ability to help more
0 -
The withdrawing groups can affect on acidity as well. Carbonyl group is almost withdrawing; therefore the CH which is placed between two carbonyl groups (proton B) is enough acidic. Also when proton B is removed the remaining carboanion can be stabilized by two carbonyl groups through resonance. the acidic nature of this proton is famous and it has been used in "base catalyzed Aldol reaction".
The proton E is related to the alcoholic group. Alcohols are not considered acidic. but it is more acidic than proton A,C and D.because the proton is attached to the electronegative atom (oxygen). after removing the proton the oxygen anion is kinda stable. but not as stable as the carboanione which can have resonance with two carbonyl.
0 -
If y = ln(x)
What is dy/dx
What therefore is delta_y in terms of delta_x
Can you relate delta_y and delta_x to standard errors?
sorry, I can not follow it. should i use the derivative of the function to calculate the error for each data?
0 -
I have some data with defined standard error. I should calculate the ln() of each data. do you know what would be the error of new data?
0 -
I have to present the attached paper in one of my course. I have too many difficulties to understand some part of that:
1- RRKM theory in this paper has been failed to apply and i do not get why?
2-in "Vectorial decomposition coordinate" section they have used reaction coordinate vector to measure the rate constant ratio. do you have any idea how they have done that? I checked the references and they were not that much helpful
0



Mendeley references
in Homework Help
Posted
Hi all,
I am writing my thesis and managing the references. I have faced an issue. For each chapter, I want to start the reference number from 1 (not starting from the last reference number of the last chapter).
I am using Mendeley desktop as reference manager and I am using microsoft word office.
I have tried "page break" and "section break" but It did not solve the problem. I appreciate any help.
Thanks