

Jacko123
-
Posts
6 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by Jacko123
-
-
but molecules do not have 2 d structures. if you want to get any meaningful results from your application, it needs to be 3d like in reality.
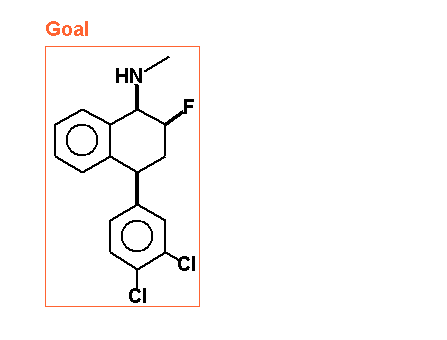
See the attached file with example of fluorosertraline. The attached file contains a molecules which can be synthesised. I want some more molecules to test my application.
Thanks
0 -
I don't think anyone has synthesised hexaphenyl ethane yet; there are plenty of similar examples where steric considerations make the molecule impossible. Still the question is a bit odd, I think that several hundred million different compounds have been synthesised. Which ones do you want big ones, smelly ones, left handed ones?
Actually I want some 2D structure of compunds that can be synthesised. I want some complex molecules that I can test on my application.
Thanks
0 -
Hi,
Can somebody out there provide some molecules which can be synthesised.
Thanks
0 -
Why can't your employer help you out?
Well, I am in constantly touch with my client and he clarifies any doubt I have. Can you please suggest me some resouces which can help me to get some fundamentals of organic chemistry. I dont want to become an expert but I think I should have basic knowledge to communicate with my employer.
How useful will my application be to chemistry guys???? Please provide some input..
Here is the description of my application
'The editor allows a chemist (or the user) to define the molecular structure for a compound and deduce various possible sub-molecule and its combination to achieve that after synthesising. This project consists of two (2) modules, a Molecule Editor and a Synthesis viewer. Molecule Editor allows the user to define the molecule structure, edit it and save it, while the Synthesis viewer allows the user to view the synthesised results. Molecule Editor generates an output file .CT or connection table information. This is the input for the synthesiser or chemistry engine, it then computes the break-up and unionization of molecules and generates a .SYN or synthesised file. Synthesis viewer loads the synthesized results in its viewer along with the %age yield in all possible paths, for the user to choose the right path to achieve the desired results.'
Thanks
0 -
Hi All,
My first post.
Basically I'm from computer background and is very new to orgranic chemistry. You must be wondering why I'm here. Well, I develop softwares for my client who are basically from chemistry background. They want me to develop a chemistry related application where the user should be able to create molecules, set varius properties, see 3D view of molecules and view the synthesis result. The application that I'm developing is quite similar to "Chemsketch" but my application has options to see the synthesised results. This means you have a goal molecule. Now to achieve that goal molecule you may have various steps to obtain. My application determines how to achieve the goal molecule with best results. The result can be based on cost, yield etc.
now I have some basic questions regarding orgranic chemistry
There are variuos options I need to make use of in my application but I dont know what those means. Before I start creating the interface I just want to make sure I know what I'm doing. Here are the options and please let me know what each means
1. "atomlimit"
2. "carbonlimit"
3. "chirallimit"
4. "functionalgrouplimit"
5. "heteroatomlimit"
6. "ringlimit"
7. "catalog"
8. "doubtlimit"
9. "reflimit"
10. "steplimit"
11. "yieldlimit"
12. "resolutionlimit"
13. "asymmetriconly"
14. "ignorechirality"
15. "selective search"
16. "industrial"
17. "deomit"
18. "omit",
19. "omitcondition"
20. "omitreactionswithleavinggroup_x"
Thanks
0

Some molecule
in Organic Chemistry
Posted
what is Web of knowledge????