-
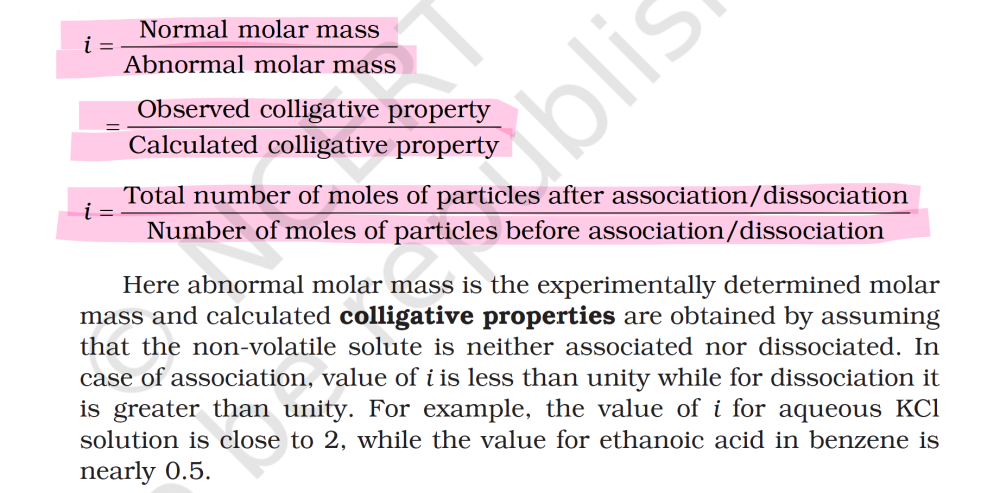
[Chem-Applied-Solutions] van’t Hoff factor i
@KJW I don't get the context of "The number of moles is inversely proportional to the molar mass." in van't hoff's factor. The definition/formula in book seems self-contradictory.
-
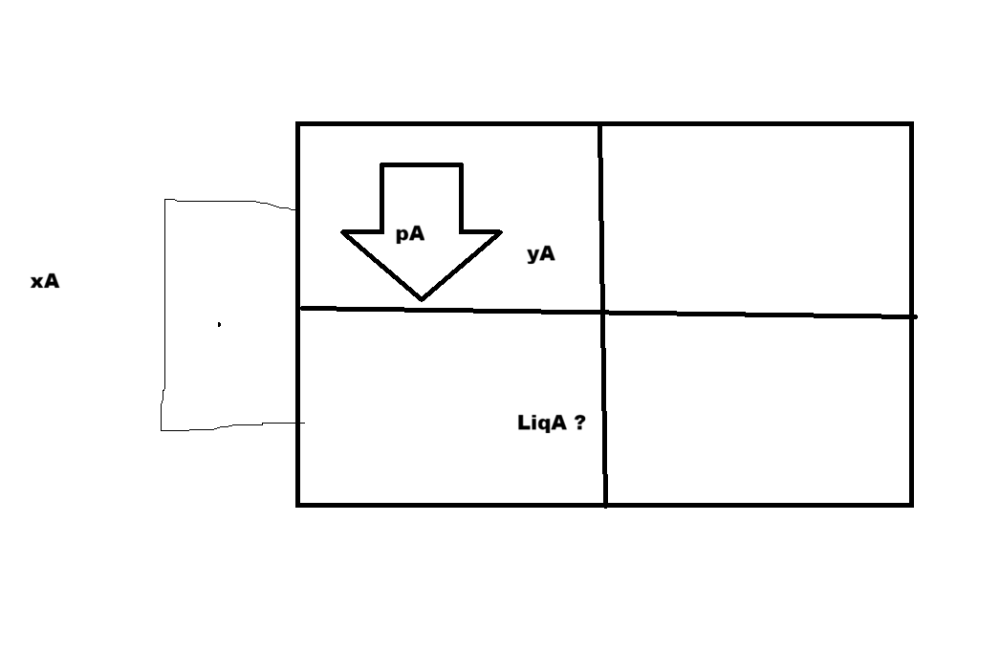
[Chem-Applied] Vapour pressure of Pure Liquids A & B are 450 & 700mmHg respect. @350 K . Find comp. of liquid mix, total Vapour pressure is 600mmHg and Vapour phase composition
I meant LiqA/LiqA+LiqB
-
[Chem-Applied-Solutions] van’t Hoff factor i
@KJW What is that have to do with ? I meant Normal = Calculated and Observed=Abnormal (has the definition below it says)
-
[Chem-Applied-Solutions] van’t Hoff factor i
-
[Chem-Applied] Vapour pressure of Pure Liquids A & B are 450 & 700mmHg respect. @350 K . Find comp. of liquid mix, total Vapour pressure is 600mmHg and Vapour phase composition
I still don't know how to find comp. of liquid mix (LiqA in diagram). it is not possible to be get the value (from the infomation in the book). or I got the question wrong ? He is my guru
-
[Chem-Applied] Vapour pressure of Pure Liquids A & B are 450 & 700mmHg respect. @350 K . Find comp. of liquid mix, total Vapour pressure is 600mmHg and Vapour phase composition
Given: p°A = 450 mm Hg (pure component's vapour pressure), °B = 700 mm Hg, = 600 mm Hg Let xA and xB are mole ractions, So xB = 1 − xA Using Raoult’s law: Total Vapor pressure = mole fraction of component A in it's vapour and liquid phase times the Vapour pressure of pure component A + same thing for B P = xA·p°A + xB·p°B 600 = 450xA + 700(1 − xA) 600 = 700 − 250xA xA = 0.40 xB = 1-.4 = 0.60 Partial vapour pressures: pA=pA^0 * xA (Parital pressure of a component = vapoyr pressure of pre component times it's mole fraction) pA = xA·p°A = 0.40 × 450 = 180 mm Hg pB = xB·p°B = 0.60 × 700 = 420 mm Hg pA and pB are vapour partial pressure, ie the pressure exerted by them on solution Vapour phase composition: pi = yi * P (partial pressure of a component = the ratio of it's vapour phase the vapour phase of the other component) times the total vapour pressure on solution yi=pi/P yA = pA / P = 180 / 600 = 0.30 yB = pB / P = 420 / 600 = 0.70
-
[Chem-Applied] Vapour pressure of Pure Liquids A & B are 450 & 700mmHg respect. @350 K . Find comp. of liquid mix, total Vapour pressure is 600mmHg and Vapour phase composition
@exchemist updated my answer Also did i get the definations right and the question ?
-
[Chem-Applied] Vapour pressure of Pure Liquids A & B are 450 & 700mmHg respect. @350 K . Find comp. of liquid mix, total Vapour pressure is 600mmHg and Vapour phase composition
@exchemist Given the context of the question AI is right but If I got the definattion right, it is not possible to be get the value (from the infomation in the book). Either I got the question wrong or the definetion. Ok the conceptual problem is - We got ratio amongst vapours and ratios of the components amonst their phases How are they connected ? It should be something like - Let V and L be phase of Vapour and LIquid in the solution V/L = [1/Q1] / [(xA/y1) + (xB/y2)]/Q2 Q is some ratio to normalize the value from 1 to a fraction of the solution - it should be y1+y2/xa+xb V/L=[1/(y1+y2/xa+xb)] / [(xA/y1) + (xB/y2)]/(y1+y2/xa+xb) V/L=[1*xa+xb/y1+y2] / [(xA*y2+xB+y1/y1y2) / (y1+y2/xa+xb)] 0.30+0.70/L=xA + xB 1/L = 0.40+0.60 1/L=1 L=1/1 V/L=ya/xa
-
[Chem-Applied] Vapour pressure of Pure Liquids A & B are 450 & 700mmHg respect. @350 K . Find comp. of liquid mix, total Vapour pressure is 600mmHg and Vapour phase composition
I worked same solution but why is AI saying it's liquid phase composition ? It's -xA and xB are mole fractions of each components - Vapour-liquid phase ?
-
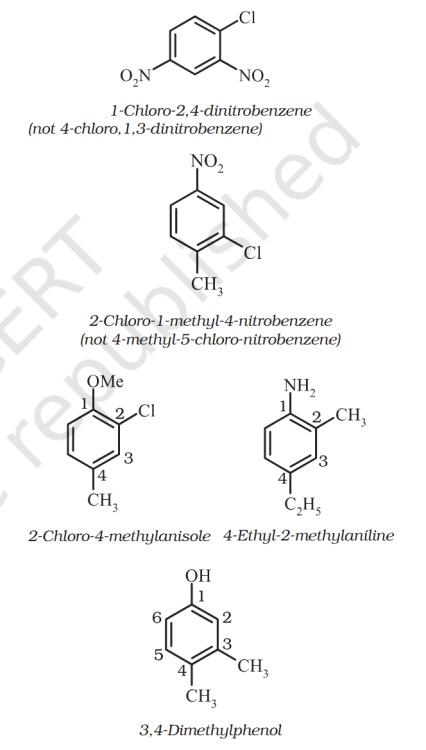
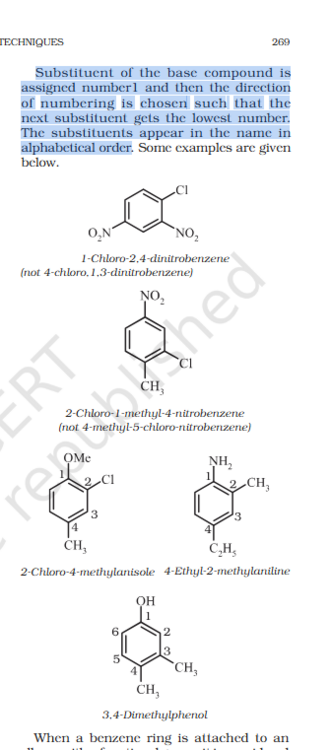
What is the Benzene base compound priority list ?
-
[Chem-Applied] Vapour pressure of Pure Liquids A & B are 450 & 700mmHg respect. @350 K . Find comp. of liquid mix, total Vapour pressure is 600mmHg and Vapour phase composition
xA and xB are mole fractions of each components - Vapour-liquid phase ? why is AI saying it's liquid phase composition ? Henry's Law : p = KH x “partial pressure of as in vapour phase (p) is proportional to ole fraction of gas (x) in solution” and is expressed as: Raoult’s Law: A=xA pA∘ pi = yi P total - this is the ratio of 2 components in vapour phase AI Solved - Given: p°A = 450 mm Hg p°B = 700 mm Hg Total vapour pressure, P = 600 mm Hg Let xA be the mole fraction of A in the liquid phase. Then xB = 1 − xA Using Raoult’s law: P = xA·p°A + xB·p°B 600 = 450xA + 700(1 − xA) 600 = 700 − 250xA xA = 0.40 Therefore: xA = 0.40 xB = 0.60 Partial vapour pressures: pA = xA·p°A = 0.40 × 450 = 180 mm Hg pB = xB·p°B = 0.60 × 700 = 420 mm Hg Vapour phase composition: yA = pA / P = 180 / 600 = 0.30 yB = pB / P = 420 / 600 = 0.70 Final Answer: Liquid phase composition: xA = 0.40 xB = 0.60 Vapour phase composition: yA = 0.30 yB = 0.70
-
What is the Benzene base compound priority list ?
How to choose the base compound ? And start counting from that ? IS there is a prioty list ?
-
Alkenes general formula ?
i think this should be the answer but everywhere i looked it doesn't have +2
-
[Organic Chemistry] Nomenclature
Proply is c3h8 not 6 but in 2nd and 3rd example in main thread, in 3rd it means - propyl mean C3H5 Iso means 1 branch of methyl on 2nd but in 1st and 2nd example it doesn't follow that @exchemist
-
Benzene with polysubstituent nomenclature.
Is the book self-contradictory or am I tripping. Also what is the order of preference for base substituent ?

HbWhi5F
Senior Members
-
Joined
-
Last visited