-
Separating & isolating reagents from product - ammonium acetate, ethanol, nitromethane, salicylic acid
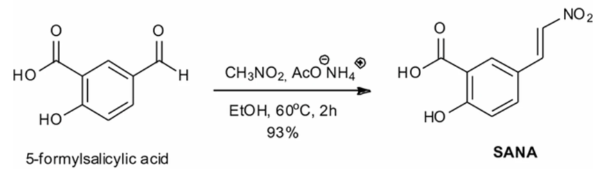
Per the researchers, their yield is 93% of theoretical.
-
Separating & isolating reagents from product - ammonium acetate, ethanol, nitromethane, salicylic acid
Hmm. Then I think that the ammonium acetate is there because when it breaks into ammonia and acetic acid in solution, the ammonia can function as a weak base to catalyze the nitroaldol reaction required to remove the NO2 group from the nitromethane.
-
Separating & isolating reagents from product - ammonium acetate, ethanol, nitromethane, salicylic acid
Yeah unfortunately the above diagram is all I have here. I've contacted the researchers inquiring as to methodology specifics so I can tinker until I manage to recreate it. Am I wrong in thinking that the format of the published reaction is a bit wrong here? ammonium acetate and nitromethane are shown above the line, where reagents, catalysts etc go. Except it looks like the nitromethane is consumed in the reaction which makes it a reactant and belonging on the left side of the equation and not above the line. Same with the ammonium acetate I think?
-
Separating & isolating reagents from product - ammonium acetate, ethanol, nitromethane, salicylic acid
So the nitromethane ends up donating its NO2, and in the presence of ammonium acetate is further reacts to end up with a negatively charged acetate and a free ammonia? Ideally 100% conversion to the salicylic acid derivative, but a little bit of unreacted product is acceptable. It's more the reactants and other end products I want to remove after. I'm checking solubility tables and it looks like just by adding water to the ethanol, the salicylic acid derivative (assuming it has similar properties) should drop out of solution, at which point I can decant and then evaporate.
-
Separating & isolating reagents from product - ammonium acetate, ethanol, nitromethane, salicylic acid
So where I've gotten thus far is: ammonium acetate's 1000x more soluble in water than salicylic acid, so any crystallizing would be the salicylic acid derivative and then mechanical separation. Adding water to the mixture will most likely cause the nitromethane to mostly separate as well, due to its poor miscibility with water - the ethanol will fully mix with the water and the nitro will mostly separate out, so a separatory vessel takes care of that (mostly). One question is where the salicylic acid prefers ethanol or nitromethane - I mean, either way I can separate it out. But given salicylic acid transforms >70*C, using distillation is out of the question, and vacuum distillation's a step more fancy than I think I can pull off.
-
Separating & isolating reagents from product - ammonium acetate, ethanol, nitromethane, salicylic acid
Hi! I am trying to solve a process for isolating the product of a reaction from the reagents. Basically there's a salicylic acid derivative created, in the presence of nitromethane, ammonium acetate, in an ethanol solution. Removing the salicylic acid at the end is easy: add water, cool, the salicylic acid drops out of suspension and crystallizes, then either filter or decant + evaporate then wash a couple times in hot water and recrystallize / evaporate to purify. HOWEVER, I believe the ammonium acetate will also drop out of suspension and crystallize, and I need to remove that prior? Or I could try crystallizing the salicylic acid, probably get a bunch of ammonium acetate crystals in the process, decant those from the nitromethane and ethanol, wash, and come up with a final step to separate from the ammonium acetate?

lexxatron
Members
-
Joined
-
Last visited