

Ertox
-
Posts
3 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by Ertox
-
-
1 hour ago, exchemist said:
I would expect it to be the same mechanism, just in 2 stages, with an alkene as the intermediate step. In both alkynes and alkenes you have π-bonds which can bind to the metal surface. Kinetically, I imagine it may be a bit faster for alkynes, as they can approach in any orientation and still bind to the surface.
There are descriptions of this on the internet. Here is one: https://www.masterorganicchemistry.com/2011/11/25/hydrogenation-alkenes-palladium-on-carbon-pdc/. This link suggests that alkynes are more readily reduced than alkenes.
The only respect in which I think the mechanism you have drawn may not be quite right is that, according to my understanding, the alkyne or alkene itself binds to the metal via its π-bonds, whereas you have shown the molecule staying above the H atoms, rather than binding to the surface itself before reacting. (There is a diagram of the mechanism in the link.)
Thank you for your prompt reply it's much clearer now, I've redone the reaction shema.
Have a nice day
Sincerely0 -
Hello everyone, I'm new to the forum and I'm french so, sorry for the mistake!
 I'm doing a presentation on an internship in which I reduced an alkane to an alkyne. To do this, we used paladium on carbon with hydrogen gas. Unfortunately, I can't find anything on the internet about this... I "imagined" this shema on the assumption that it was the same as for alkenes, but it doesn't really seem professional. If anyone can help me, I'd really appreciate it.
I'm doing a presentation on an internship in which I reduced an alkane to an alkyne. To do this, we used paladium on carbon with hydrogen gas. Unfortunately, I can't find anything on the internet about this... I "imagined" this shema on the assumption that it was the same as for alkenes, but it doesn't really seem professional. If anyone can help me, I'd really appreciate it.
Thanks !
0

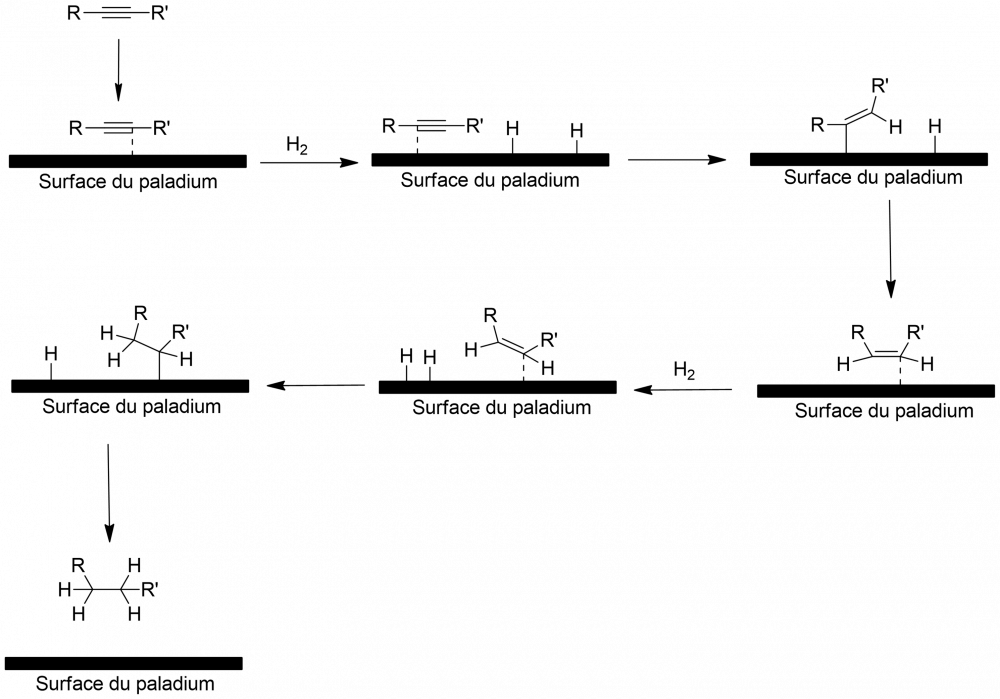
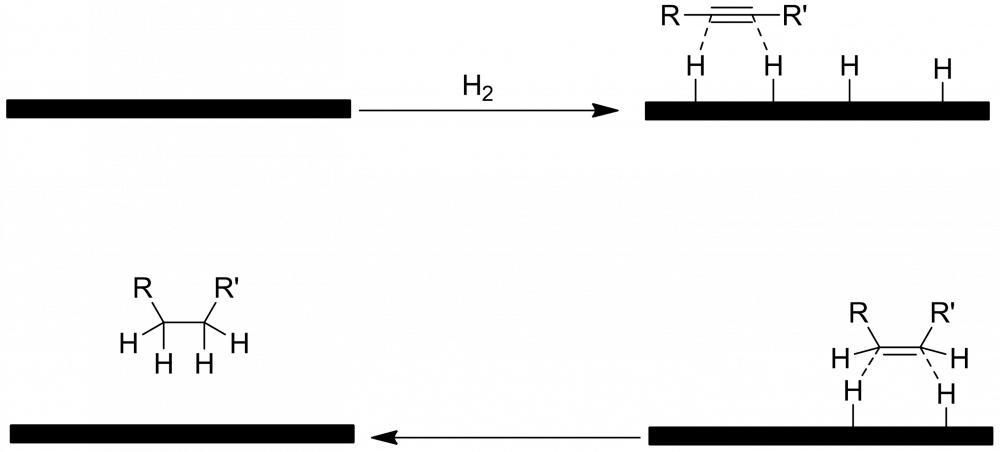
catalytic hydrogenation of an alkyne
in Organic Chemistry
Posted
No, I don't think I'll be looking into that. I'll just point out that the addition is cys.