

mundane
-
Posts
24 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by mundane
-
-
So the other day I had a circular tub filled with water. I added a few grams of detergent to it and started stirring with my hands. As expected, detergent tended to settle right at the center of the tub. Was this because of centripetal force? In washing machines, the clothes are spinning but are pushed away from the centre due to centrifugal force. I am not sure. Can someone help me understand the forces acting in both the scenarios?
0 -
I just learned that there are three kinds of overlapping (positive, negative and zero). I don't thoroughly understand the concept of different signs in orbitals and their overlappings. What does difference would it make if the signs are different (negative overlapping happens) when they don't really play a significant role? Could someone explain how waves and orbital shapes along with overlapping in brief?
0 -
-
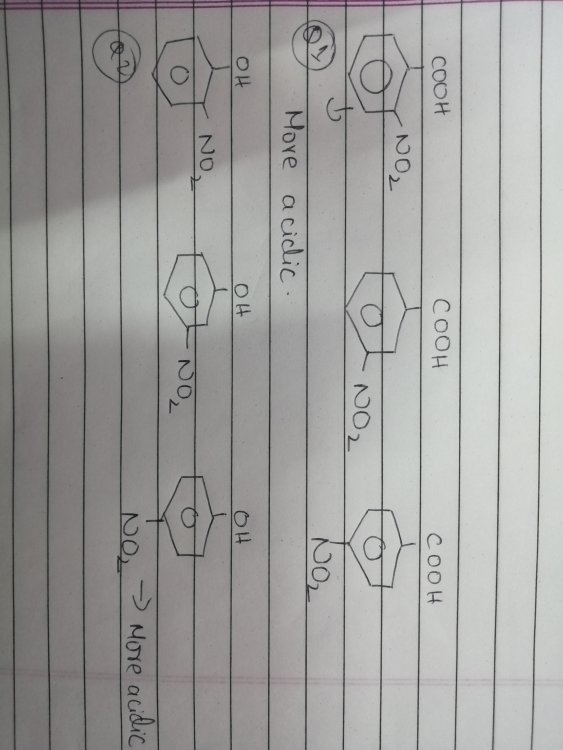
As you see in the first question -NO2 on ortho causes inductive effect and creates a hydrogen bonding with H in COOH and is considered to be stronger acid than others. In the second question, the -NO2 again shows I- and H bonding but is less acidic. I don't get how the latter one isnt the strongest.
0 -
-
I was wondering why we need substances which contain acid in acidity. Can someone help me out?
0 -
the mole ratio of acid: salt in production of an acidic buffer solution is between 1/10 to 10/1.
5mol HCOOH + 5mol KOH isn't a buffer solution, why?
1mol HCOOH + 1mol HCOOK is a buffer solution, why?
0 -
-
great explanation yet I failed to understand as to why COOH being the strongest couldn't overcome the inductive effect of Cl. please clarify!
0 -
-
3 minutes ago, iNow said:
I'm not sure it does, but if we assume this is true then almost certainly it's because the lotion or petroleum jelly do a better job of holding on to the volatile scent compounds and molecules than bare skin does. They evaporate off the bare skin more rapidly than the lotion or vaseline where they're more likely to remain "caught."
could you be more specific? like how do alcohol and oil work together?
0 -
why does the fragrance of the perfume last longer if we spray it on the part of our body where we have applied non fragrant lotion or vaseline?
0 -
3 hours ago, Strange said:
The key thing is, can the two forms be made the same by one or more rotations.
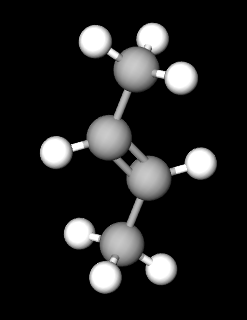
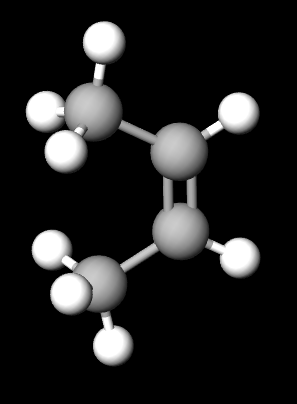
So in your G1, if you were to create an isomer by swapping the R and H at the top, then you could just rotate it by 180° to get back to G1.
In G3, if you were to swap the orientation of the diether ring you can still rotate the whole molecule by 180° and get back to where you stated.
But in G2 if you swap the R and H at the top, you can't rotate it to get back to the molecule you started with. You have to reflect it. In other words, you have a chiral pair (like a pair of gloves, rather than a pair of socks).
If this is not clear from looking at the diagrams, then you need to build a model as Sensei suggests.
(I bought a ball and stick kit when I was doing chemistry but never used it because I can see the symmetries immediately from the drawings. Which is bizarre because I have almost total aphantasia and can't tell left from right!)
oh! now I get it! thanks alot!
1 hour ago, Ghideon said:@mundane you could use the ideas above in digital tools* if you prefer. Here is an example of chloromethane (did not have time to create an animation of it in rotation)
Here is an example view of two types of But-2-ene. You cant rotate one to get to the other.
*) There are free tools available online
thanks for the explanation, bud!
1 -
13 minutes ago, studiot said:
It's basically the same reason why there are no isomers of chloromethane.
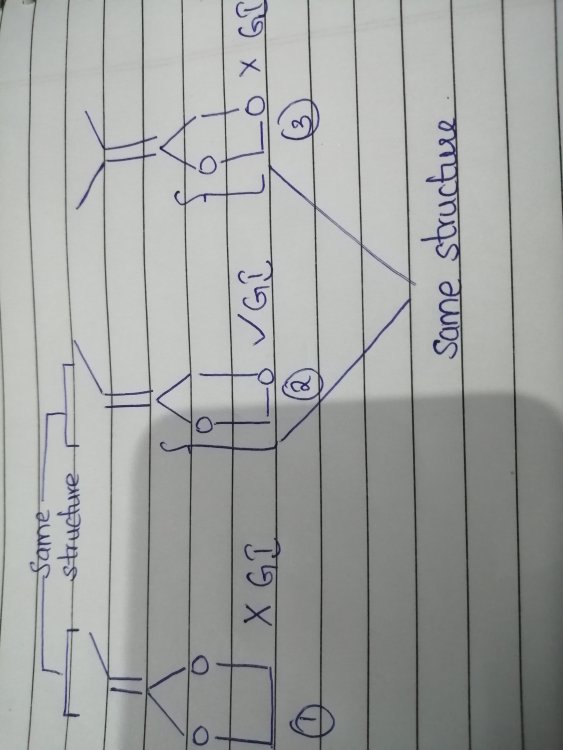
Your G1 and G2 are not the same structure.
In G1 the diether ring is symmetrical, in G2 it is not.This means that which ever way round you attach the H and R groups, to G1, you can rotate the molecule to achieve the other configuration.
In G2 there is no rotation that will achieve this.In G1 and G2, one R and one H are attached on the top, yet only G2 shows isomerism.
The diether ring in G3 isn't symmetrical either, yet it doesn't show GI.
0 -
19 minutes ago, studiot said:
I thought a picture was worth 1000 words ?

The left hand one corresponds to your left hand one.
The (top) end has one hydrogen and one alkyl group as shown.
Swopping these over generates a different isomer since the R group is now closer to the nearest oxygen.In the right hand picture swopping two identical R groups does not alter the relative disposition of constituent atoms - they are the same molecule from front and back.
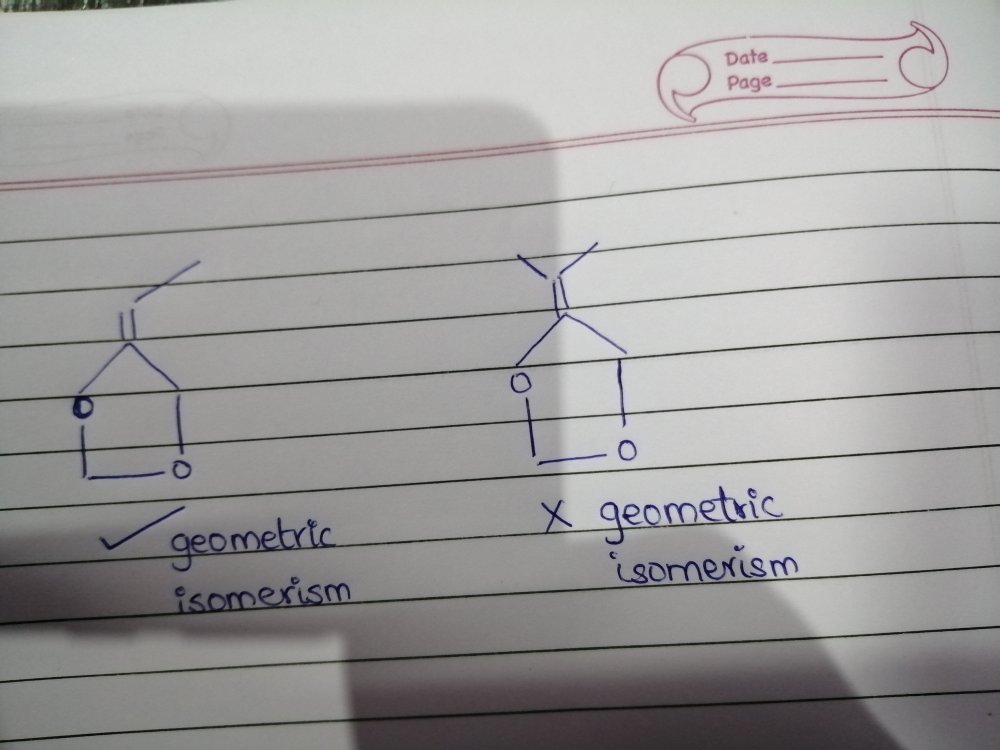
in that case, please look at figure 1 and tell me why can't it show GI while figure 2 can.
0 -
sort of. can you explain?
0 -
-
Why is that isopropyl alcohol and s-butyl alcohol having just -CH2 difference, have different naming?
0 -
in that case finding radius of isolated atom shouldn't be a big deal
0 -
if it's difficult to isolate an atom then how do we find wave function of an isolated one? is it just a hypothesis?
0 -
so I put it in this way- I have read in that we can't isolate an atom like to find its radius. like, hey, come here alone, lil guy, I have to measure your radius. We always measure the distance between two nuclei of two atoms and divide the it by 2.
on the other hand, in wave functions we take two or more isolated independent atoms.
Ψ=Ψa+Ψb
Ψa- atom a Ψb- atom b
so my question is when we can get an atom isolated then why can't we take out its radius without having it been bonded (covalent, metallic and ionic)?
0 -
if we can isolate an atom then why do we first find the distance between nuclei of two atoms and divide it by 2?
0 -
why is it not possible to isolate an atom to measure its radius? There are many works in chemistry which require isolated atom for example in wave functions.
0


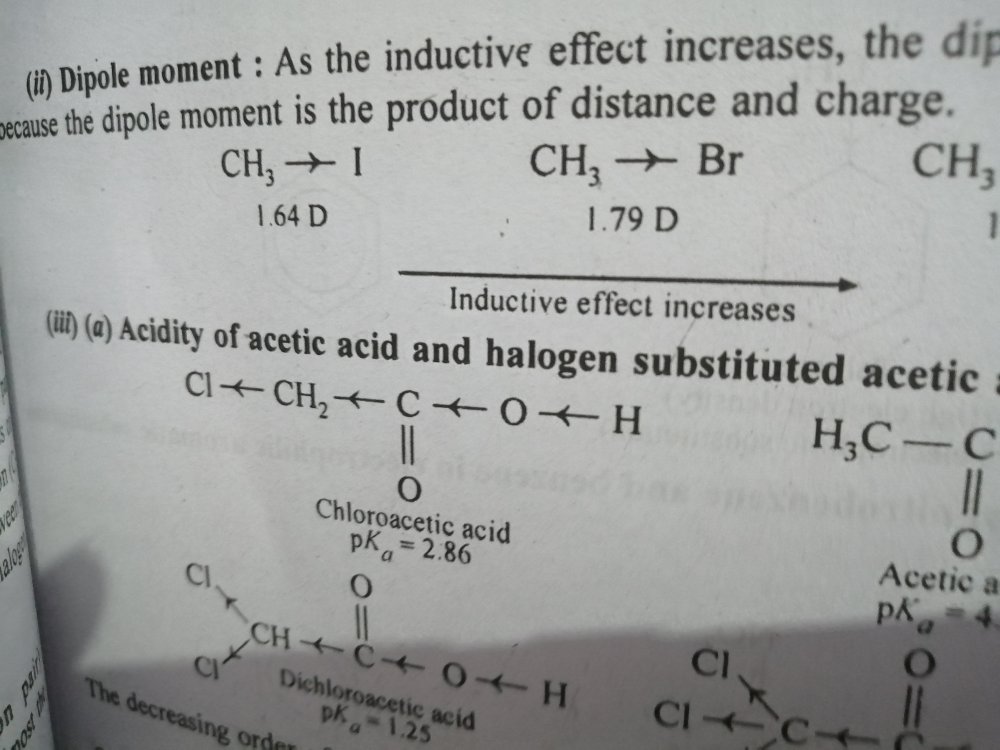
lewis acid
in Inorganic Chemistry
Posted
So, i just learned that because of backbonding in BF3, BF3 is a weak acid. This is due to the fact that B orbital if filled internally with F electrons. Now, if we bring NH3 to make a coordinate bond with BF3, it does. Why? Wasn't the vacant orbital of B already filled during backbonding? I am confused.