

Jeremiah23
-
Posts
3 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by Jeremiah23
-
-
1 hour ago, hypervalent_iodine said:
I’d say your main problem is that you can’t hydrolyse the ester with an alkoxide like that.
You need a hydroxide, or you need to do it under acidic conditions.
I really appreciate your reply. Why can’t this reaction be done in basic condition? I’m still not very clear in your statement.
0 -
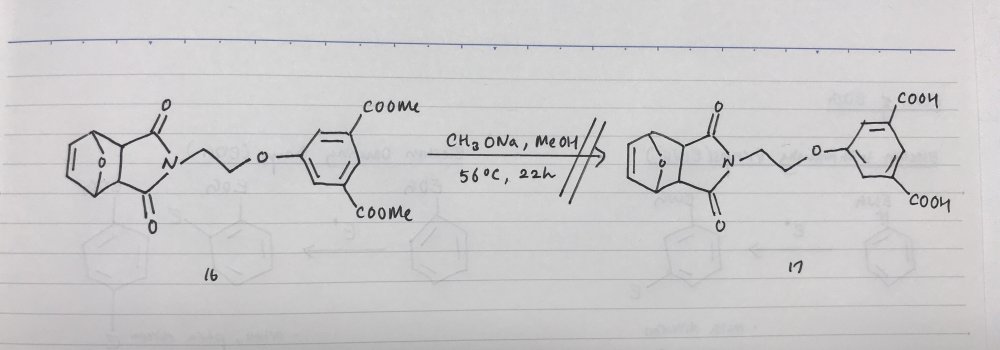
Hi. I am currently doing a research project on synthesizing a water-soluble rotaxane via Diels-Alders reaction. But I am facing a problem in synthesizing this molecule(please refer to attached photo) which is part of the process to synthesize a dienophile for Diels-Alder reaction. I anticipated to be able to synthesize compound 17 from compound 16 via hydrolysis of the methoxycarbonyl group, but it went unsuccessful. I think instead of the methoxycarbonyl group, the maleimide group underwent hydrolysis. Are there any ways to resolve this issue? How can I hydrolyze the methoxycarbonyl group under the presence of maleimide?
0

Esterification
in Organic Chemistry
Posted
Hello.
I am just wondering whether it is possible to combine the hydroxy group of 4-Hydroxy-1-naphthalenesulfonic acid sodium salt to another compound with -COOH group via esterification? If so, what are the appropriate reagents used for this?