

Sci101
-
Posts
17 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by Sci101
-
-
I would like to know on whether the latest status about "spooky action at a distance" is confirmed on how it work or still exploring what it is.
Do you have any suggestions?
Thanks, to everyone very much for any suggestions (^v^)0 -
I need to watch more youtube to learn about Quantum Mech before reply
Thanks, to everyone very much for suggestions (^v^)
0 -
29 minutes ago, John Cuthber said:
Thanks for confirming that I was right.
You do not know what you are talking about.
Go and learn.
Here's a start
https://en.wikipedia.org/wiki/Molecular_orbital
Magnesium glycinate contains the same types of atoms as are present in chlorophyll.
But it is white.
So it is clear that the colours of compounds don't come from atoms.
What you found in the CRC book is the emission spectrum of the O++ ion which does not exist in any chemical compound. The header in the book tells you what the I, II III etc signify.
It's just that you are too lazy to read.
Why Leaf is GREEN that is topic to be discussed, if you can explain it clearly, that is great, any reference is not directly related to the topic, it just change the FOCUS. Could you please briefly explain on how molecular orbital related to GREEN leaf?
Thanks, to everyone very much for any suggestions (^v^)
0 -
4 hours ago, John Cuthber said:
I suggest that you learn some science and some humility.
The atoms in chlorophyll are carbon, oxygen, nitrogen, magnesium and hydrogen.
None of those is green.
So looking at it on an atomic level you would think that chlorophyll isn't green.
I am polite, but too straight on communication and keep learning to talk with more human feeling.
Referring to CRC Handbook of Chemistry and Physics,
Oxygen has intensity 1000 on both 508.182 and 525.795 wavelength, which is green, but I don't know on what III represented on reference.
I cannot confirm on whether the intensity is strong or not, but GREEN wavelength is existed on chlorophyll based on your source.
What about go back to the discussed issue? (learning to be more human :>)
Thanks, to everyone very much for any suggestions (^v^)
1 hour ago, dimreepr said:At the size of a photon, there is space between the atoms and the majority of photons that get to the other side of the leaf is reflected light hence green.
When above image refer to reflected light, could you please provide on what reference / theory is? and I try to understand more on your viewpoint.
Thanks, to everyone very much for any suggestions (^v^)
-3 -
35 minutes ago, StringJunky said:
Swansont has a PhD in atomic physics and the Bohr model is so last century. It's now a curiosity from a time long since passed.
Ha, you just change the FOCUS, so what theory he applies to describe better than Bohr model on how Chlorophyll absorbs blue and red light and not GREEN in atomic levels?
Do you have any suggestions?
Thanks, to everyone very much for any suggestions (^v^)0 -
1 hour ago, swansont said:
Because there are no transitions with energy differences corresponding to green light, involving states that are populated.
Your statement is not related to any view point from science.
so if you do not discuss on atomic level related to the Bohr model, you would miss the key point.
Do you have any suggestions on how thing works?
Thanks, to everyone very much for any suggestions (^v^)0 -
There is exactly back to original question again, it just change the FOCUS,
1) Leaf absorbs blue and red light,
2) now we look closer that Chlorophyll absorbs blue and red light
There is still missing explanation on how Chlorophyll absorbs blue and red light and not GREEN in atomic levels.
Do you have any suggestions on how thing works?
Thanks, to everyone very much for any suggestions (^v^)
Chlorophyll absorbs blue and red light:The green pigment, chlorophyll, plays a central role in photosynthesis. The fact that it is green means that it absorbs blue and red light and reflects green when it is illuminated by white (all wavelengths) light.http://happy-firewalker.blogspot.com/2015/03/where-does-protein-come-from-photons.html
0 -
48 minutes ago, swansont said:
No, you should not be looking at the law for different media. In the same medium (air), the reflection angle is the same as the incident angle.
You see the green because the red and blue are not reflecting. Those colors are absorbed. They do not bounce.
The light you see is not emitted light. It is reflected light.
Read my responses, and stop trying to force your misconceptions into the answer. The Bohr model is only a small conceptual part of the explanation - that some light is absorbed.
There is a missing explanation area, what cause red and blue colors to be absorbed, and not GREEN in atomic levels. I would like to know on how thing works.
Do you have any suggestions?
Thanks, to everyone very much for any suggestions (^v^)0 -
1 hour ago, swansont said:
The "bounce" in the video is absorption by a virtual state. The emission follows certain rules (same energy, follows Snell's law, which is conservation of momentum. No energy or momentum imparted to the atom.) The blue and red absorptions in a leaf are in real states. The photons do not "bounce" — they do not follow the law of reflection, because energy and momentum must be transferred to the atom.
Referring to following video, your mentioned Snell's law (for different media) not applied on why leaf is GREEN, Sunlight shines on leaf through air, and we see GREEN leaf through air (same media here). Since you agreed that photons do not "bounce", which must be transferred to the leaf's atom. Why do Leaf only emission GREEN light instead of other colors? if you do not agree on the Bohr model.
Do you have any suggestions?
Thanks, to everyone very much for any suggestions (^v^)0 -
Referring to following video, I would like to know on why leaf only bounce off GREEN light and not other colors on atom levels.
Do you have any suggestions?
Thanks, to everyone very much for any suggestions (^v^)0 -
5 hours ago, Strange said:
Stop ignoring explanations.
QuoteFor example, in green plants, the action spectrum resembles the absorption spectrum for chlorophylls and carotenoids with absorption peaks in violet-blue and red light. In red algae, the action spectrum is blue-green light, which allows these algae to use the blue end of the spectrum to grow in the deeper waters that filter out the longer wavelengths (red light) used by above ground green plants. The non-absorbed part of the light spectrum is what gives photosynthetic organisms their color (e.g., green plants, red algae, purple bacteria) and is the least effective for photosynthesis in the respective organisms.
I would like to understand on how each step works, "in red algae, the action spectrum is blue-green light" and in ground green plants, the action spectrum is red light.
When Light pass from sky to ocean, Ground green plants absorb red light to grow (let assume somehow red light can be absorbed, that is not the main discussed issue), how green plants handle blue light in atom levels? why is leaf GREEN, not BLUE? where do BLUE light go?
Do you have any suggestions?
Thanks, to everyone very much for any suggestions (^v^)0 -
3 hours ago, Strange said:
You are still focussing on the behaviour of single atoms being excited by a photon. There are other mechanisms. The energy of the photon is enough to cause the chlorophyll molecule to lose an electron - it is this free electron that starts the chain of reactions that make up photosynthesis. The chlorophyll molecule eventually gets its electron back from another source (when a water molecule is split by another molecule). The chlorophyll molecule doesn't release a photon when it gets its electron back and so there is a net absorption of red/violet photons.
More here: https://en.wikipedia.org/wiki/Photosynthesis#Light-dependent_reactions
" In the light-dependent reactions, one molecule of the pigment chlorophyll absorbs one photon and loses one electron." Leaf is GREEN (that is FACT), electron must drop from upper orbit to lower orbit in order to emit GREEN wavelength photon (energy between different orbit's levels = GREEN wavelength energy / photon), that is why we see that leaf is GREEN. Since the distance between orbits is fixed (let assume), Leaf - element's orbit structure cannot absorb BLUE wavelength photon, because the energy between orbit's levels are different. How can chlorophyll absorb BLUE / RED wavelength photon from this viewpoint? Do BLUE / RED wavelength photon just pass through Leaf without any interaction with any electron within orbit from leaf element?
Do you have any suggestions?
Thanks, to everyone very much for any suggestions (^v^)0 -
2 hours ago, swansont said:
Actually chlorophyll absorbs more strongly in the red and blue, which is why we see green.
When chlorophyll absorbs red and blue, the electrons along the orbits must absorbed exact red and blue wavelength energy and jump up to another orbits, that is called absorption. However, the electrons on upper orbits do not go up forever and would drop down to original orbits and emit red and blue, I would like to know on why leaf is green instead of red and blue, if you mention that chlorophyll absorbs more strongly in the red and blue.
Do you have any suggestions?
Thanks, to everyone very much for any suggestions (^v^)0 -
Leaf is green, green energy wavelength with white light is absorbed by any leaf's element, which change electrons into higher energy levels and then electrons drop back to lower energy levels and emit green wavelength, so we see Green Leaf. would it be correct process to see color ?
Do you have any suggestions?
Thanks, to everyone very much for any suggestions (^v^)0 -
14 hours ago, swansont said:
https://en.wikipedia.org/wiki/File:Water_infrared_absorption_coefficient_large.gif
As you can see, liquid water absorbs red more strongly than blue. So white light will be preferentially absorbed in the red end of the spectrum, leaving water looking blue. Things in the water can also absorb light, changing the color.
Referring to your linked image, wavelength shows up to 100 um, which is outside the visible light. If water absorb red, then water must emit red instead of blue based on changing energy levels. Do you have any reference source to show water absorbing red more than blue?
Furthermore, x-axis is for wavelength. I would like to know on what object is measured on the y-axis of the chart,
Do you have any suggestions?
Thanks, to everyone very much for any suggestions (^v^)0 -
Referring to following video, it seems that for Balmer Series there are many elements dropping to n = 2 orbit, which release blue color wavelength. When blue wavelength is scattering, it requires electrons to interact with 2nd orbits to absorb and radiate energy (blue color wavelength). That make Ocean looking blue from space.
Furthermore, for following orbits, they release infrared wavelength.
Paschen Series (3rd Orbit) - Infrared wavelength
Brackett Series (4th Orbit) - Infrared wavelength
Pfund Series (5th Orbit) - Infrared wavelength
It seems that color / wavelength are fixed based on absorb and radiate energy levels. so why apple is red? which orbit levels are related to absorb and radiate energy (red color wavelength)? is the distance between orbit levels fixed within N2 (Blue) and O2 (Yellow or Orange) structure?
Furthermore, when red color wavelength do not change any orbit levels within N2 or O2 atom, does red color wavelength just pass through atom without any interaction with atom's electrons?Does anyone have any suggestions?
Thanks in advance for any suggestions0



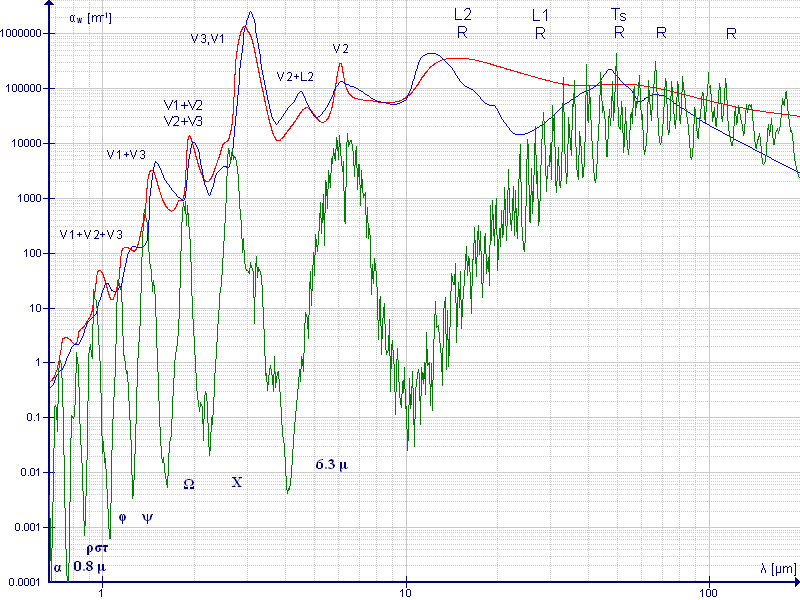
What elements make Ocean Blue from Space?
in Quantum Theory
Posted
D
Do you have any specific keywords related to atom under QM? so I can search for more specific topic under QM on this issue.
Do you have any suggestions?
Thanks, to everyone very much for any suggestions (^v^)