

Chu
-
Posts
20 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by Chu
-
-
Hello,
I am working on a potentiometric titration lab for a diprotic phosphoric acid. Attached is the excel document of my lab.
We are diluting 1.022 M NaOH by a factor of 5 and then using it to titrate with 25 ml 0.06M Phosphoric acid in a beaker.
I know my titration curve is correct, however my TA told me that the scaling of my x-axis is wrong for my first and second derivative curves. I know that I need to use the average volume. I also know that I take must take the raw volumes and correct for the initial reading in order to find the average, but I am not exactly where I am going wrong.
What I am doing is taking the second volume and adding it to the first and dividing it by 2.
Such that it looks like, (as taken from the spreadsheet):
(A4+A5)/2 or (3.88+6.3)/2
If someone would be willing to help me out that would be great!
0 -
I used the ka to find the pka and got 4.202.
15 mL * 0.1 M = 1.5
25 mL * 0.1 M = 2.5
I then used the equation
pH=pka+log([base]/[acid])
=4.202+log(2.5/1.5)
=4.42
0 -
The pH of a mixture of 15 mL of 0.1 M NaOH with 25 mL 0.1 M HBZ is:
Ka=6.28x10-5
The correct answer is 4.38, but I keep coming up with 4.42.
If someone could should me how to get to 4.38 that would be greatly appreciated.
Thanks!
0 -
I see what I did wrong. I didn't do parenthesis first and went to multiplication.
0 -
A chameleon can accelerate its tongue in order to catch an insect at an impressive rate of 500m/s^2. How quickly must a fly located at 5cm from the chameleon react if it is to escape?
Given:
Vi = 0
Vf = ?
xi = 0
xf = 5 cm
a = 500m/s^2
Using the equation:
Vf2=Vi2+2a(xf-xi)
Vf=70.71m/s
Could someone please double check this answer for me.
Thanks!
0 -
I thought that you'd like to know that I figured some of it out!
In order to find the [HCl] (M) - STOCK I had to use this equation for trial 1:
(((0.1298 g Na2CO3*10)*(1 mol/105.9888 g)*(2 mol HCl/1 mol Na2CO3)+(25.75 mL NaOH used*(1 L/1000 mL)*(2.052 M NaOH STOCK/ 1 L)))/0.05 L
0 -
Okay, I will do that ASAP!
Thank you for all your help it's really appreciated.
0 -
I haven't done it yet. Everything but the gray comes pre filled and we are expected to learn it and turn it in before we can do the lab. I pretty much gave you everything they gave me. The only thing I did was subtract initial volume from final.
0 -
I got the endpoint from the mL of total NaOH used. G10-G12 from the picture.
0 -
I tried what you said, but I think I am missing something. For trial 1 these were my results:
0.1298g Na2CO3 yielded 0.0024493 mol HCl used the first reaction. Then converted I 25.75 mL NaOH to 0.052839 mol NaOH given the M of 2.052 from the stock.
Since I didn't know the concentration of HCl I added 0.0024493 + 0.052839 = 0.0545332 mol HCl
I then divided 0.0545332 by 0.05L (from the 50 mL HCl used and ended up with 1.091M HCl.
This yield is too small of a number to fall within one standard deviation of the number in the blue box in the picture and therefore cannot be right. I even tried to solve for the other 2 trials and found the average to be 1.112.
0 -
You mean a balanced chemical equation like this?
Na2CO3 + 2HCl → 2NaCl + CO2 + H2O
0 -
I don't think I am following you. All I can think of is using a density equation which I know is incorrect. I don't see how the mL of HCl is suppose to convert into an equation with the Na2CO3 that would result in the molarity of HCl used.
0 -
For the first table NaOH is being titrated from the buret into a erlenmeyer flask containing the Na2CO3, DI water, and 50 mL HCl (which is of course the flask is boiled first). Then it is titrated to the endpoint. The results are as follows in the excel image.
Calculations I have tried (this is all they have taught us to use):
g of Na2CO3 to find g of HCl and then attempting to find the M of HCL used and stock, but I just get stuck after g HCl
(stock NaOH * Vol NaOH used)/(****)/(vol flask/vol pipet) (found this one online and I feel like its the closest but don'd know how to apply it)
((2.052M * 25.75 mL)/****)/(249.74/24.996)
I think what I am really getting stuck on is the dilution part between the stock solutions and the 10x dilution of 25 mL into a 250mL vol flask, as I have never done this before and now I am running out of time to do the assignment. It is due tomorrow (Sunday) at 5 PST.
I forgot to mention that I have tried other equations, I just forgot to write them down as I did them in excel and quickly found out that they were wrong. And as for table 2 it is just a standard direct titration between NaOH (buret) and HCl (flask).
0 -
I have tried but every attempt leaves me with incorrect answers so I just left those blank for the purpose of this forum. Everything in the gray is what I have/know how to fill out. The teachers aren't telling us students anything and are kind of just throwing us out here with no idea what to do really.
0 -
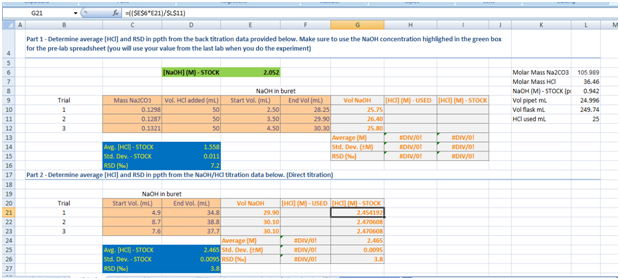
I need help filling in the values for [HCl] (M) - USED (for both parts) and [HCl} (M) - STOCK (for part 1)
More specifically I need to know the equations and the inputs (like the area in the fx writing space) that would result in my average, standard deviation, and RSD % resulting in numbers that would match the blue boxes.
0 -
Thank you!
0 -
A car travelling at 30mph accelerates at a rate of -10 miles per hour per second. How long does it
take to come to a stop? What is the car’s average speed during that time? How far does the car travel
during that time? (b) A car travelling at 60mph accelerates at the same rate. How far does it travel
before coming to a stop? How does this compare to your answer for part (a)?
Can someone please show me how to solve this. I know the answers, I just want to know how to perform this problem.
Thanks!
0

Double Checking Answer for Finding Final Velocity
in Classical Physics
Posted
I was using the wrong equation!
using
Xf=1/2at2
0.05=1/2(500)*t2
0.05=250*t2
t2=0.0002 square this to get:
t=0.014 s