-
Posts
56 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by nancy9494
-
-
-
very helpful thank you!
0 -
I've looked at some school requirements and all they say is 2 years of undergrad lab work. Some-kind of experience is better than no experience I guess.
thanks
0 -
Hey,
I'm majoring in microbio and I want to pursue a PhD/MD. Does it matter what type of research I do as an undergrad? I am trying to get into a microbio lab but the spots are limited, but there are some spots open in some biomedical labs. Would it look weird if I got a position there?
0 -
ahh yes thank you
0 -
the OH
 0
0 -
The water because it is under acidic conditions?
0 -
-
-
I just don't understand why it can't chill in 3rd form. I can memorize the mechanism but I'd prefer to know.
 0
0 -
-
-
awesome thanks!
0 -
-
out here saving lives! thanks again
 0
0 -
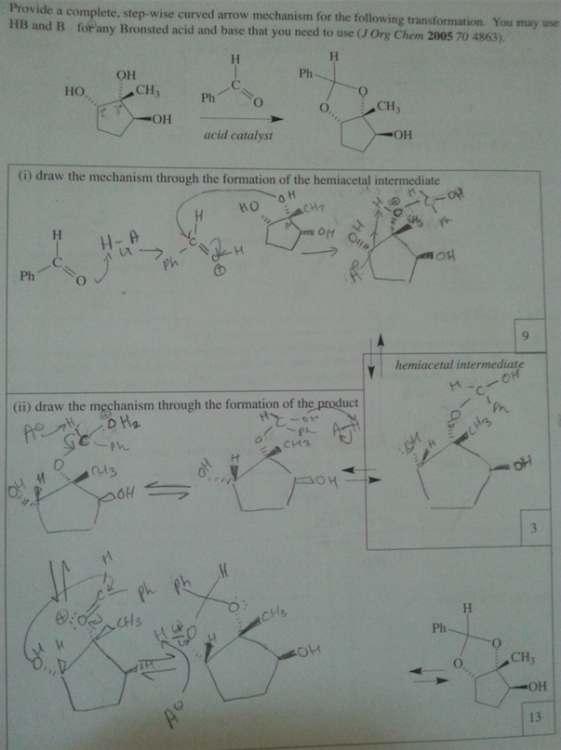
Never mind about this one. The professor went over it and the second step H3O+ is missing.
The first one OH deprotinated by the CH3 then protonated by the the H3O+.
Excess same as first but the another CH3 attacks the ketone and that is later protonated by H3O+ [The added CH3 is on a wedge]
0 -
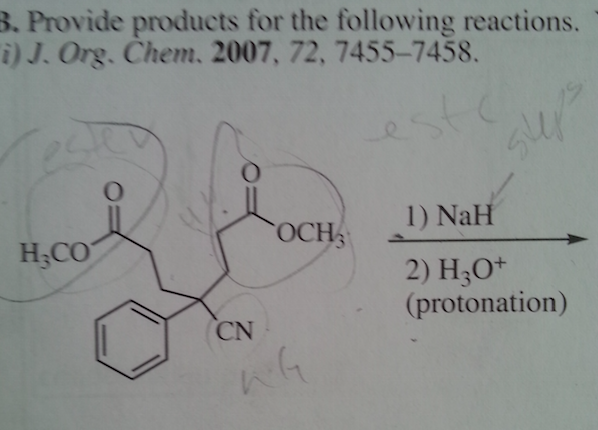
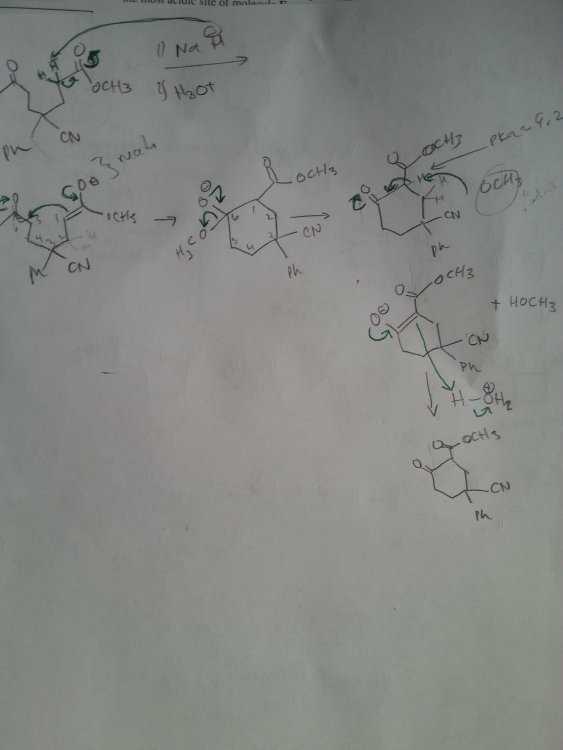
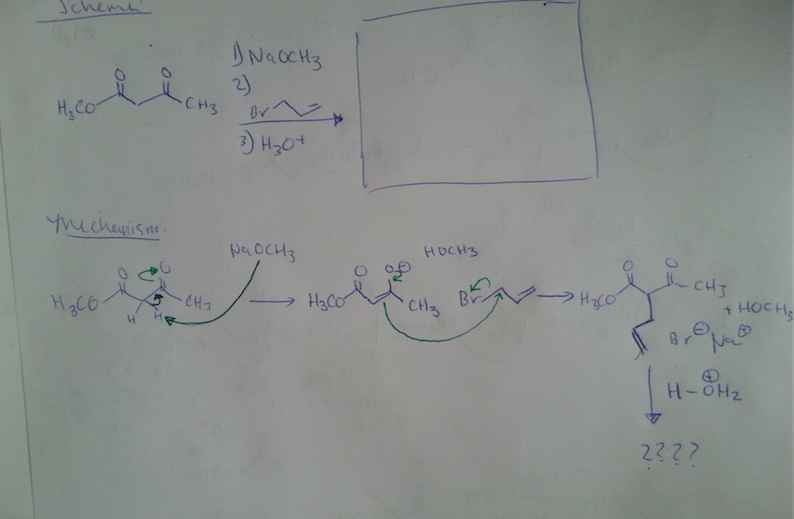
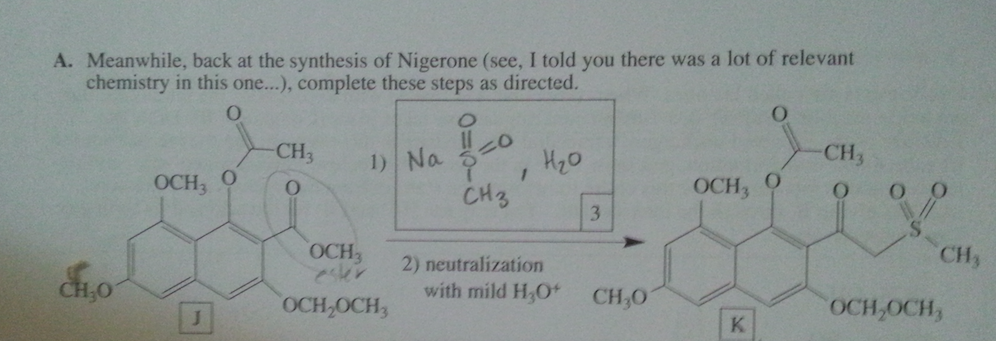
What is a supporting information document? Is that in the journal? We are not allowed to use references for the exam. I think they just want us to use our tool bag of Orgo 2 knowhow. It is supposed to be doable without it. sigh. I asked my GSI (graduate student instructor) and she said the first step is deprotination of the OH group. So I'm guessing if there's excess the C anion will deprotinate the OH and attack the ketone. The formal charges of the O's make me uncomfortable.
0 -
part ii) If the OH group is protonated with an acid, it can be kicked off by the nucleophilic oxygen when it forms a double bond with the C.
Would it react with the OH that are on dashes faster because they are closer together and most of this process is intramolecular?
Thank you so much for your help. I really appreciate it.
0 -
-
-
-
as always thanks again!
 0
0 -
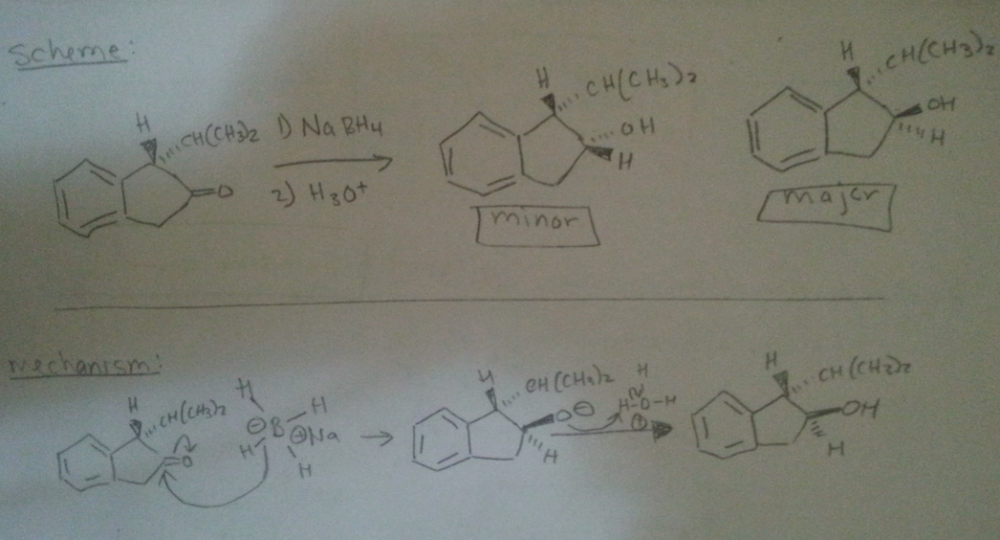
I'm confused. Shouldn't the larger substituents be opposite to each other? So the OH has to be oriented away from the CH(CH3)2. Did I label them incorrectly then? If so, is it because the strong nucleophile of Hydride anion is irreversible so the path of least resistance [front side attack] will determine the major product formed?
0 -
hello,
I wanted to know if i labeled the possible products correctly [i.e. minor vs. major].
reasoning: Although there is less steric hinderance for the Hydride ion through front (because of sterics of the CH(CH3)2 group on the dash) that won't be deciding factor for the amount of major product formed. The back side attack will form the major product because the larger OH and CH(CH3)2 groups are anti to each other. Is this correct?
thank you in advance
0



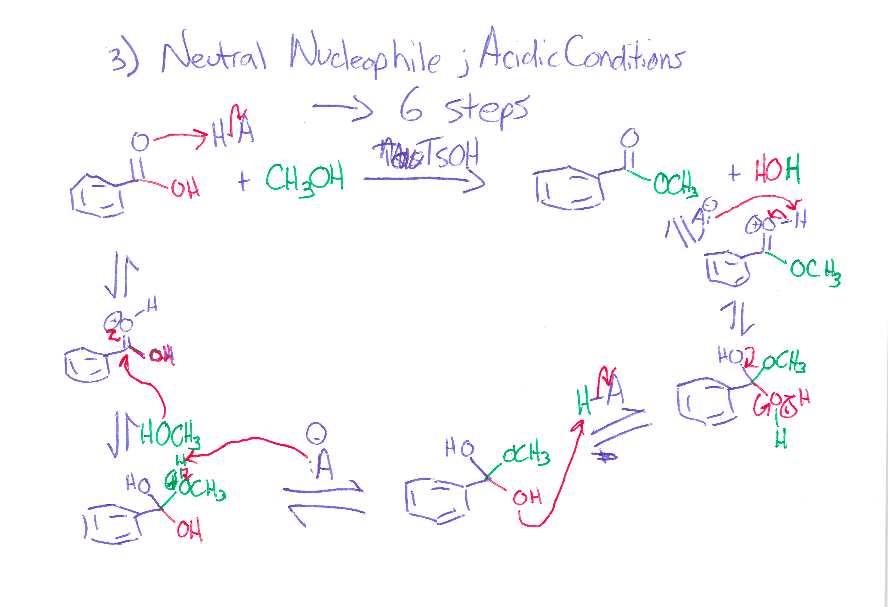

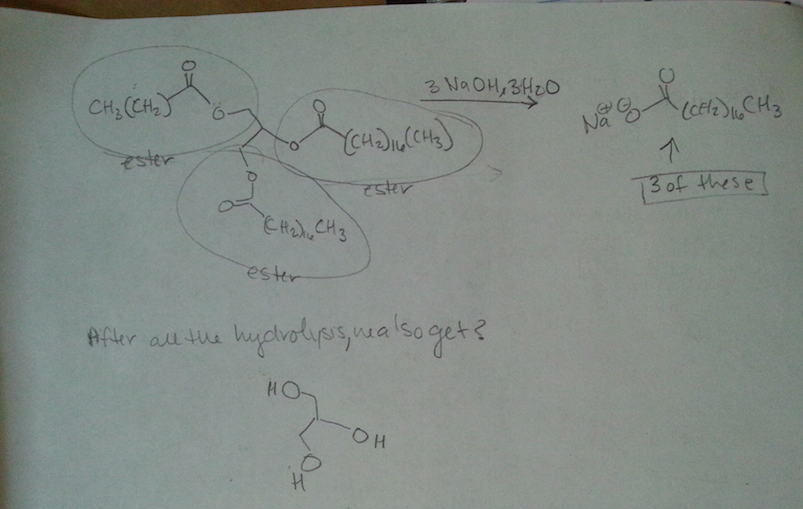
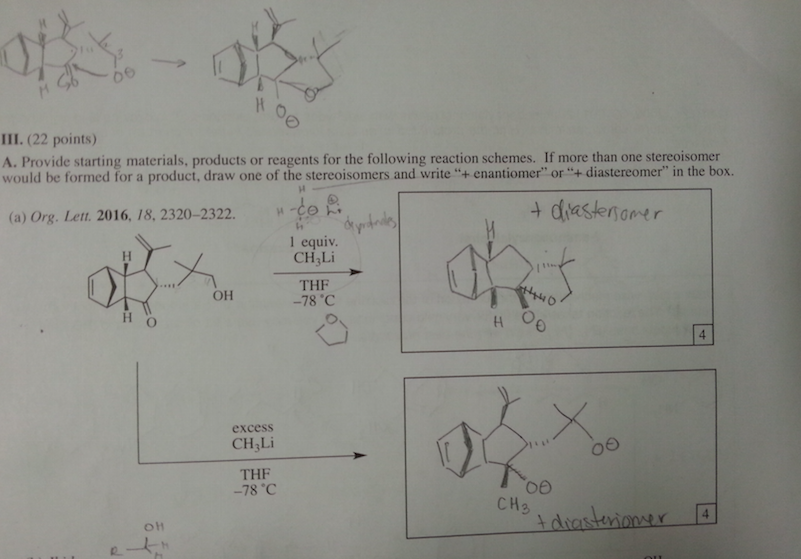
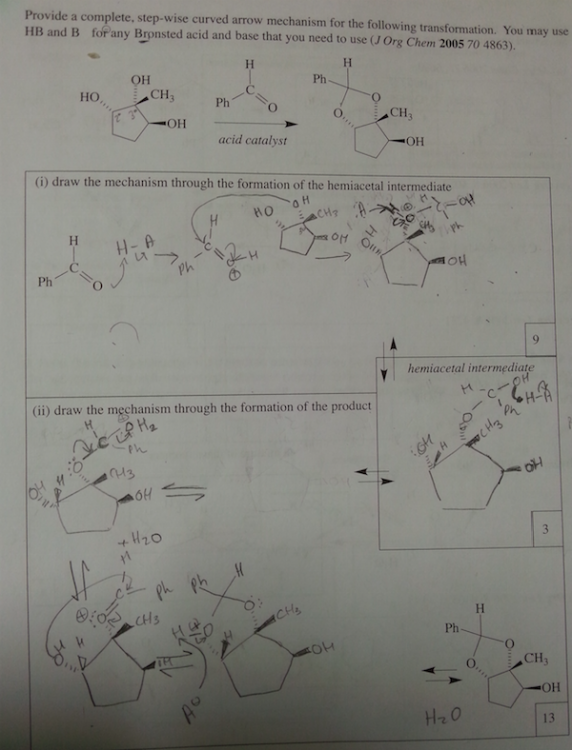
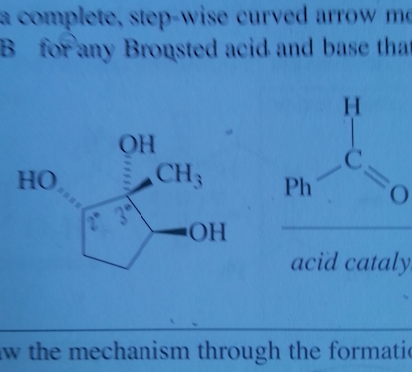
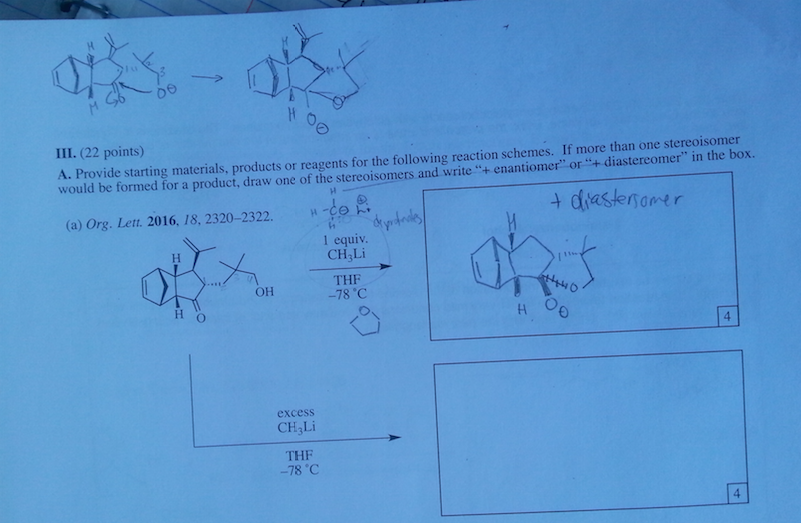
Diels-Alder reactions
in Organic Chemistry
Posted
Hello friends
I dont understand how the end product is the answer. I have labeled the partial charges. Did I do them wrong? My reasoning is that the ph are EDG.
thank you in advance