

whitesniper001
-
Posts
15 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by whitesniper001
-
-
Anyone have any input, would be very grateful
 0
0 -
-
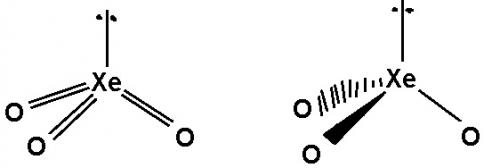
According to VSEPR theory would I be right to assume that the first image is more accurate rather than the second one as it includes the double bonds present which can’t be shown using wedges and dashes? Thanks
Also when drawing a molecule using VSEPR do you show lone pairs that are not on the central atom but on another atom? Or do you only show lone pairs on the central atom and ignore the rest but include them only when drawing a lewis dot structure? Thanks
0 -
Cheers for the books presented will try to get a hold of some of them. You are right I hardly do use books mostly its based off watching youtube videos, going through articles and lecture notes.
0 -
Cheers again, looked through all the files presented.
0 -
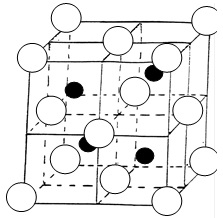
Just going through lattice structures, was finding it hard to identify the number of spheres in unit cell, the two which I struggled with were:
- I know that this lattice represents a compound such as ZnS
- The coordination number is 4:4 for both white and black spheres.
- The number of white spheres (face centred cubic lattice) in the unit cell is: (1/8 x 8) + (1/2 x 6) = 4
- The black spheres (tetrahedral holes) I am having problems with, my guess is: 1 x 4 = 4
The reason I calculated it to be 4 is because only half the tetrahedral holes are filled, normally for a structure like Na2O there are 8 white spheres inside and they are all counted as entirely inside. So if there are 8 white spheres and they are counted as (1 x 8), would the same logic then apply if there are only 4 white spheres?
My question is, have I calculated the unit cells for the black spheres right if not where have I gone wrong?
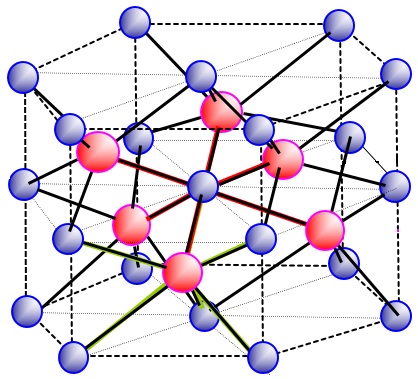
The second lattice I was really struggling with was:
- I know the coordination number is 6:6
How do I calculate the number of unit cells in the above lattice? It is the most complicated one I have come across so far.
0 -
Thanks for the useful direction.
0 -
Had a question relating to Schematic diagrams, just going through a past paper and the following two question came up:
- What is peculiar about these structures?
- Why are these structures stable?
For Question 2, after having gone through my notes I answered:
Layer structures are intermediate between:
- A totally ionic crystal with regular arrangement of ions and strong electrostatic forces.
- A crystal in which small discrete molecules are held together by weak forces such as van der Waals forces and hydrogen bonds. Hence the reason for their stability.
I am stuck on Question 1 that asks "What is peculiar about these structures?". The only answer that I came up with after having gone through my notes is that they have " repulsion between layers which is normally shown by the double headed arrows in-between them."
Since I don't have much to go by and I find It hard to even find information regarding this, are my answers valid? Or have I completely gone off track? Any input would be greatly appreciated.
0 -
Thanks for the reply, just read up on the topic some more and you input helped.
0 -
Thanks for the clarification I understand now what you mean by it being cumulative and not a point function. I've being reading "Chemical, The Central Science by Brown" which seems like a more introductory book. I will try getting hold of a copy by Moore also. Thanks
0 -
Thanks for the posted pages, have read them and referred to another book also which has helped make the issue alot clear, thank you.
0 -
You haven't indicated the level of science or maths this question is for.
Because the (electrostatic) potential energy of an electron is a function only of its distance from the nucleus; that is the force field is spherically symmetrical, it is possible to express the total wave fucntion as a product of three functions, each involving only one of the three coordinates in a spherical coordinate system.
Two of these coordinates are angles and the third is the radius.
Thus
[math]\psi = R\left( r \right)\Theta \left( \theta \right)\Phi \left( \phi \right)[/math]
Where capitals represent the function and lower case represents the variables.
Theta and phi are the angles, r is the radial distance from the nucleus.
For all s electrons the angular part is constant for all values of of the angles.
This is because the s orbitals correspond to the zero value for the subsidiary quantum number, l
So all s orbitals have spherical symmetry, so only the radial part R® need be considered.
The probability of finding an s electron in a small element of volume dv at a distance between r and (r+dr) from the nucleus is then
[math]{\left[ {R\left( r \right)} \right]^2}dv[/math]
This is the same regardless of the direction (ie the angles) of the radius vector.
The total probability of finding an electron anywhere between r and (r+dr) is found by integrating the expression over a spherical shell between r and (r+dr)
ie replacing dv by the total volume of the shell.
This volume is
[math]4\pi {r^2}dr[/math]
So the integral now in terms of r becomes
[math]4\pi {\left[ {R\left( r \right)} \right]^2}{r^2}dr[/math]
This function is known as the radial probability distribution, which is plotted on your y axis.
Are you with me so far?
Should have indicated this before asking the question, but I am a first year undergraduate chemistry student. I do follow you so far as it similar to what my notes contain. So the y axis is used to calculate the total probability of finding an electron anywhere between r and (r+dr) from what I gather.
0 -
I assume the quote from your question was a misprint and you do indeed mean radial, not radical.
That makes sense.
Yes they are the correct graphs.
Important features.
Well maxima or minima are usually important featuresof interest in a graph.
Alice the camel has 2 humps.
How many humps has the 1s, 2s and 3s?
Are they all the same size?
What is plotted on the axis
X axis?
Hint hence the radial distribution
Y axis ?
Note the function plotted is (proportional to) the square of the wave function - What does this signify?
What do you thing the r2 is doing in the y axis function?
Yes sorry that was suppose to say radial and not radical.
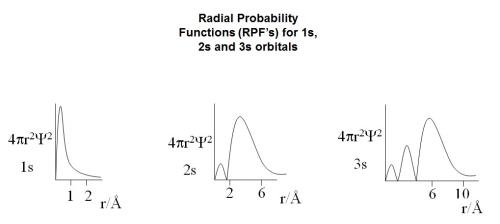
1s has one hump
2s has two humps
3s has three humps
The humps are not all the same size. The nodes present in the graph what do they mean?
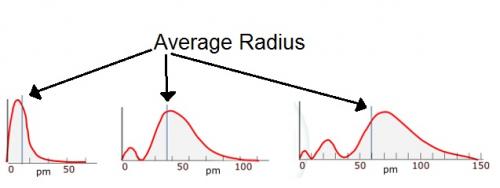
Is the average radius plotted on the x axis?
Does the square of the wave function signify the probability of finding an electron? Also I know that r represent the distance from the centre of the nucleus so would r2 signify the size of the orbital?
I have uploaded some more graphs indicating the "average radius", why is it that these lines are not exactly half way between the final hump?
0 -
Hi, just needed some help in answering a past exam paper question which is as follows:
"Draw the Radical Distribution Functions for 1s, 2s and 3s orbitals and point out the important features of these graphs".
After looking through my notes the graphs which I found that correspond to the "Radical Distribution Functions " are :
First question Is are these the right graphs? Even though they are titled "Radial Probability Functions (RPF’s)".
Also there was no information regarding the second part of the question in regards to the "important features of these graphs." I tried looking online and was finding it hard to find anything, can anybody point me in the right direction? Thanks
0

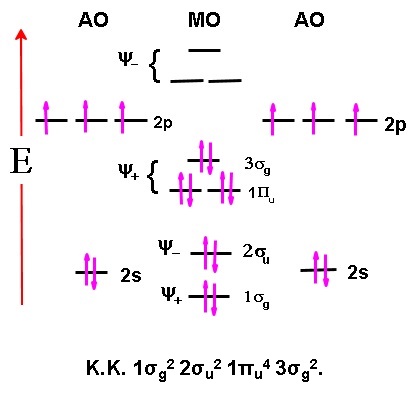
Question about KK for molecular orbital diagrams.
in Homework Help
Posted
Anyone have any input, would be very grateful