

Suzy Tran
-
Posts
9 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by Suzy Tran
-
-
-
I suggest drawing the first carbocation in the reaction and asking what kind of carbocation it is (primary, secondary, or tertiary). Carbocation rearrangements occur when an electron pair from a sigma bond moves to form a different bond. They are often seen when they lead to a carbocation of greater stability in the product than in the reactant.
Well I tried drawing the mechanism and this is what I did, I think my main problem is that I can't visualize the structure of the cyclopentane turning into cyclohexane properly.
0 -
Have you learned anything about how carbocations can rearrange?
Yes, I've learned it and I actually tried to do a rearrangement as well but was unsure which bond i should move.
And.......... what exactly is preventing you from looking it up?
I just looked it up, but I am still confused about it how this can be applied to the mechanism part. I think the product from this reaction is has an internal alkene since the C=C pi bond is not at the end of the carbon chain. Also the internal alkene is more stable than the terminal alkene due to the greater number of carbons connected to the double bond.
0 -
No I am not familiar, I didn't learn that yet.
0 -
Hi guys, I need help with this E1 reaction's mechanism. I just need someone to explain what do I have to move around after the leaving group leaves.
Thanks!
PS. You can use this site to draw it and take a screenshot if you can: https://epoch.uky.edu/ace/public/mechmarvin.jsp
0 -
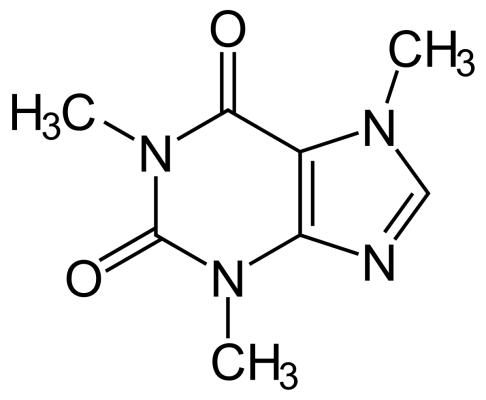
Oh I see, caffeine does have a dipole moment I think I'm not fully understanding what dipole-induced-dipole is. Then the caffeine/DCM interactions would be dipole-dipole since both are dipole?
0 -
According to my TA, there are London Dispersion but I'm unsure if both of caffeine/DCM and caffeine/water interactions go through them as the dominant interaction. In DCM, I'm pretty sure there are dipole moment between Carbon and Chlorine. One chlorine is pointing up and the other one is pulling more downwards, these two dipole moments doesn't cancel each other out so it makes DCM kind of/ partially polar. For the other interactions between water and caffeine, well there's definitely london dispersion and hydrogen bonding, I'm not sure what else so I guess those are the two for water/caffeine interactions. I guess now I'm not 100% sure about caffeine/DCM interactions. Thanks BabcockHall.
0 -
Hello I have a few problems with figuring out intermolecular forces between interactions of caffeine and water/ caffeine and methylene chloride (DCM) for my lab. Here are the questions:
- Identify and discuss two different structural features that would account for solubility in methylene chloride (hint: think intermolecular forces).
- Identify (circle on your structure) and discuss two different structural features that would account for solubility in water (hint: think intermolecular forces).
I attempted the problems and think that London dispersion forces are in between the caffeine and DCM interactions but not in the caffeine & water interactions because caffeine and water are already polar. Also, another interaction between caffeine & DCM is dipole-induced-dipole because DCM is nonpolar and is being induced by a polar compound (caffeine). For caffeine & water, I think that there is hydrogen bonding involved because the oxygens on caffeine (a part of the amide group) can interact with water for hydrogen bonding. However, I'm unsure with my answers and not sure what structural features go along with what I said especially for the interactions between caffeine and DCM.
Thank you for those that take the time to help!
0

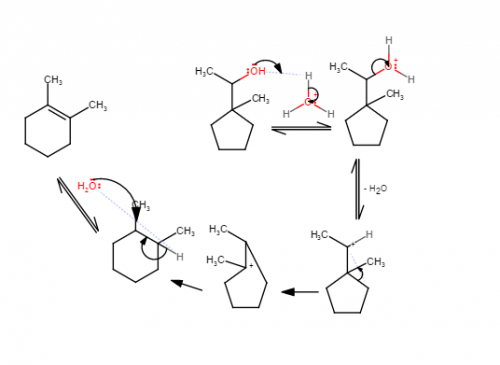
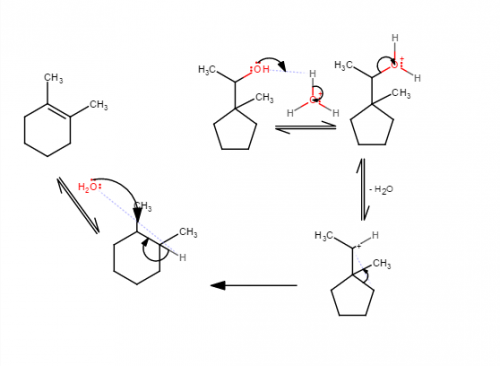
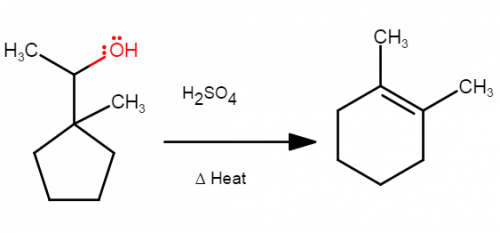
Help with mechanism steps
in Organic Chemistry
Posted
Ah thank you, I was trying to draw the distorted figure so I forgot about the steps after that. But here's what I did now.